Abstract
Purpose
To create normative data in children from binocular multifocal ERG (mfERG) recordings and compare results with the macular thickness.
Methods
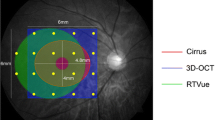
Forty-nine 5- to 15-year-old healthy, full-term children were examined with Espion Multifocal System, using DTL electrodes. The stimulus matrix consisted of 37 hexagonal elements. Amplitudes, implicit times and response densities (presented in three rings) of the first-order component P1 were analyzed. Measurements of macular thickness were performed with spectral-domain Cirrus OCT.
Results
There were no significant differences between right and left eyes regarding mfERG recordings. Median P1 implicit times of Rings 1–3 of the 46 right eyes were 30.0, 30.0 and 30.8 ms and response densities 20.5, 10.9 and 7.6 nV/deg2, respectively. Implicit time was longer in boys than in girls (p = 0.009, 0.039, 0.005 in Rings 1–3) and was correlated with age (r s = 0.417, 0.316, 0.274 in Rings 1–3). Implicit time in Ring 1 correlated significantly with the inner circle of the OCT measurements (p = 0.014).
Conclusion
Binocular mfERG with DTL electrodes is a reliable test of the central macular function in children and correlates with macular structure. As previously not shown, there was a significant difference in implicit time between boys and girls.






Similar content being viewed by others
References
van Genderen M, Riemslag F, Jorritsma F et al (2006) The key role of electrophysiology in the diagnosis of visually impaired children. Acta Ophthalmol Scand 84:799–806
Sutter EE, Tran D (1992) The field topography of ERG components in man—I. The photopic luminance response. Vision Res 32:433–446
Hood DC, Greenstein V, Frishman L et al (1999) Identifying inner retinal contributions to the human multifocal ERG. Vision Res 39:2285–2291
Hood DC, Frishman LJ, Saszik S et al (2002) Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci 43:1673–1685
Sisk RA, Leng T (2014) Multimodal imaging and multifocal electroretinography demonstrate autosomal recessive Stargardt disease may present like occult macular dystrophy. Retina 34:1567–1575
Praidou A, Hagan R, Newman W et al (2014) Early diagnosis of Stargardt disease with multifocal electroretinogram in children. Int Ophthalmol 34:613–621
Kretschmann U, Bock M, Gockeln R et al (2000) Clinical applications of multifocal electroretinography. Doc Ophthalmol 100:99–113
Andreasson S, Gosh F (2014) Cone implicit time as a predictor of visual outcome in macular hole surgery. Graefes Arch Clin Exp Ophthalmol 252:1903–1909
Molnar A, Holmström G, Larsson E (2015) Macular thickness assessed with spectral domain OCT in a population-based study of children: normative data, repeatability and reproducibility and comparison with time domain OCT. Acta Ophthalmol 2015(93):470–475
Hood DC, Bach M, Brigell M et al (2012) International Society For Clinical Electrophysiology of Vision. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124:1–13
Dawson WW, Trick GL, Litzkow CA (1979) Improved electrode for electroretinography. Invest Ophtalmol Vis Sci 18:988–991
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report. Arch Ophthalmol 103:1796–1806
Holm K, Lövestam Adrian M (2012) In diabetic eyes, multifocal ERG reflects differences in function between the nasal part and the temporal part of the macula. Graefes Arch Clin Exp Ophthalmol 250:1143–1148
R Core Team (2014). R: A language and environment for statistical computing. BM Corporation, Armonk, NY. R version 3.0.1 R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org. Accessed 10 Mar 2015
Mohidin N, Yap MK, Jacobs RJ (1997) The repeatability and variability of the multifocal electroretinogram for four different electrodes. Ophthalmic Physiol Opt 17:530–535
Hennessy MP, Vaegan (1995) Amplitude scaling relationships of Burian-Allen, gold foil and Dawson, Trick and Litzkow electrodes. Doc Ophthalmol 89:235–248
Hébert M, Lachapelle P, Dumont M (1995–1996). Reproducibility of electroretinograms recorded with DTL electrodes. Doc Ophthalmol 91:333–342
Curcio CA, Sloan KR, Kalina RE et al (1990) Human photoreceptor topography. J Comp Neurol 292:497–523
Verdon WA, Haegerstrom-Portnoy G (1998) Topography of the multifocal electroretinogram. Doc Ophthalmol 95:73–90
Fulton AB, Hansen RM, Moskowitz A et al (2005) Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc Ophthalmol 111:7–13
Jackson GR, Ortega J, Girkin C et al (2002) Aging-related changes in the multifocal electroretinogram. J Opt Soc Am A Opt Image Sci Vis 19:185–189
Seeliger MW, Kretschmann UH, Apfelstedt-Sylla E et al (1998) Implicit time topography of multifocal electroretinograms. Invest Ophthalmol Vis Sci 39:718–723
Hansen RM, Moskowitz A, Fulton AB (2009) Multifocal ERG responses in infants. Invest Ophthalmol Vis Sci 50:470–475
Hendrickson A, Possin D, Vajzovic L et al (2012) Histologic development of the human fovea from midgestation to maturity. Am J Ophthalmol 154:767–778
Seiple W, Vajaranant TS, Szlyk JP et al (2003) Multifocal electroretinography as a function of age: the importance of normative values for older adults. Invest Ophthalmol Vis Sci 44:1783–1792
Gerth C, Sutter EE, Werner JS (2003) mfERG response dynamics of the aging retina. Invest Ophthalmol Vis Sci 44:4443–4450
Ozawa GY, Bearse MA Jr, Harrison WW et al (2014) Differences in neuroretinal function between adult males and females. Optom Vis Sci 91:602–607
Munaut C, Lambert V, Noël A et al (2001) Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol 85:877–882
Marin-Castaño ME, Elliot SJ, Potier M et al (2003) Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol Vis Sci 44:50–59
Dion LA, Muckle G, Bastien C et al (2013) Sex differences in visual evoked potentials in school-age children: What is the evidence beyond the checkerboard? Int J Psychophysiol 88:136–142
Azad R, Ghatak U, Sharma YR et al (2012) Multifocal electroretinogram in normal emmetropic subjects: correlation with optical coherence tomography. Indian J Ophthalmol 60:49–52
Holm K, Larsson J, Lövestam-Adrian M (2007) In diabetic retinopathy, foveal thickness of 300 μm seems to correlate with functionally significant loss of vision. Doc Ophthalmol 114:117–124
Fujinami K, Zernant J, Chana RK et al (2015) Clinical and molecular characteristics of childhood-onset Stargardt disease. Ophthalmology 122:326–334
Eksandh L, Kohl S, Wissinger B (2002) Clinical features of achromatopsia in Swedish patients with defined genotypes. Ophthalmic Genet 23:109–120
Kjellström S, Vijayasarathy C, Ponjavic V et al (2010) Long-term 12 year follow-up of X-linked congenital retinoschisis. Ophthalmic Genet 31:114–125
Dale EA, Hood DC, Greenstein VC et al (2010) A comparison of multifocal ERG and frequency domain OCT changes in patients with abnormalities of the retina. Doc Ophthalmol 120:175–186
Acknowledgments
We thank Eva Nuija for efficient help and for performing the OCT measurements in the study. We also thank Marcus Thuresson, Statisticon AB, for valuable help with the statistical analyses. The study was supported by the Crown Princess Margareta Foundation for the Visually Impaired, Ögonfonden, and the Sigvard and Marianne Bernadotte Foundation. All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Statements of human rights
The tenets of the Declaration of Helsinki were strictly adhered to during the course of the study.
Informed consent
Written consent was obtained from the parents of the participating children and oral consent from children. Ethical approval for the study was obtained from the Ethics Committee of Uppsala University.
Rights and permissions
About this article
Cite this article
Molnar, A.E.C., Andreasson, S.O.L., Larsson, E.K.B. et al. Macular function measured by binocular mfERG and compared with macular structure in healthy children. Doc Ophthalmol 131, 169–176 (2015). https://doi.org/10.1007/s10633-015-9513-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-015-9513-y