Abstract
Purpose
Invasive lobular carcinoma (ILC) is a histological subtype of breast cancer that is predominantly estrogen receptor alpha (ER)-positive (+) and is thus treated with endocrine therapies. Herein, we sought to understand the molecular underpinnings of the 4-hydroxytamoxifen (4OHT) resistance in ILC by assessing the potential role of the epithelial-to-mesenchymal transition transcription factor (EMT-TF) SNAIL (SNAI1).
Methods
Using a series of breast cancer cell lines, we measured the basal, estrogen and 4OHT-induced expression of SNAIL and other EMT-TF family members by quantitative reverse transcription-polymerase chain reaction and immunoblotting. Chromatin immunoprecipitation experiments were performed to assess ER binding to the SNAIL promoter. Cell proliferation, cell cycle and apoptosis were assessed in 2D cultures. 3D growth was assessed in Matrigel and Collagen I cultures.
Results
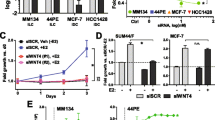
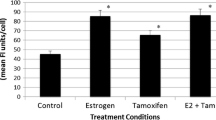
Estrogen and 4OHT induced SNAIL expression, but not that of the other EMT-TF family members SLUG (SNAI2) and SMUC (SNAI3), with the 4OHT effect being specific to the lobular but not the ductal subtype. We observed estrogen and 4OHT-induced ER recruitment to the SNAI1 promoter and high endogenous basal levels of SNAIL and several EMT-TFs in ILC cell lines. While SNAIL knockdown had a minor impact on the 4OHT partial agonism in estrogen-depleted conditions, it led to a surprising increase in cell proliferation in full serum. In complementary experiments, inducible SNAI1 overexpression caused decreased proliferation, associated with a cell cycle arrest in G0/G1. Additionally, apoptosis was observed in BCK4 cells.
Conclusion
These data suggest a previously unrecognized role for SNAIL in ILC, substantiating a context-dependent behavior for this EMT-TF.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.





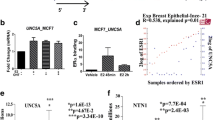
Similar content being viewed by others
References
Howlader N, Krapcho NA, Miller M, Bishop D, Kosary CL et al SEER Cancer Stat Rev. 1975–2014. 2017 April 2017; Based on November 2016 SEER data submission]
Society AC (2017) Cancer Facts and Figures 2017
Barroso-Sousa R, Metzger-Filho O (2016) Differences between invasive lobular and invasive ductal carcinoma of the breast: results and therapeutic implications. Ther Adv Med Oncol 8(4):261–266
Ciriello G et al (2015) Comprehensive molecular portraits of invasive lobular breast cancer. Cell 163(2):506–519
Christgen M et al (2016) Lobular breast cancer: clinical, molecular and morphological characteristics. Pathol Res Pract 212(7):583–597
Desmedt C et al (2017) Transcriptomic and genomic features of invasive lobular breast cancer. Semin Cancer Biol 44:98–105
Malhotra GK et al (2010) Histological, molecular and functional subtypes of breast cancers. Cancer Biol Ther 10(10):955–960
Arpino G et al (2004) Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 6(3):R149–R156
McCart Reed AE et al (2015) Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res 17(12):12
Johnson K, Sarma D, Hwang ES (2015) Lobular breast cancer series: imaging. Breast Cancer Res 17(94):94
Sobinsky JD et al. (2013) Unusual metastatic patterns of invasive lobular carcinoma of the breast. Case Rep Oncol Med 2013:986517
Shakoor MT et al (2014) Unique presentations of invasive lobular breast cancer: a case series. Int J Biomed Sci 10(4):287–293
Mathew A et al (2017) Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd 77(6):660–666
Lumachi F et al (2011) Endocrine therapy of breast cancer. Curr Med Chem 18(4):513–522
Chen Z et al (2017) Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS One 12(9):e0182397
Jordan VC (2014) Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer 21(3):R235–R246
Milani A et al (2014) Overcoming endocrine resistance in metastatic breast cancer: current evidence and future directions. World J Clin Oncol 5(5):990–1001
Smith BN, Bhowmick NA (2016) Role of EMT in metastasis and therapy resistance. J Clin Med. 5(2):17
Pestalozzi BC et al (2008) Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol 26(18):3006–3014
Narbe U et al (2014) Invasive lobular carcinoma of the breast: long-term prognostic value of Ki67 and histological grade, alone and in combination with estrogen receptor. Springerplus 3:70
Engstrøm MJ et al (2015) Invasive lobular breast cancer: the prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology 66:409–419
Adachi Y et al (2016) Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer 16:248
Araki K, Miyoshi Y (2017) Mechanism of resistance to endocrine therapy in breast cancer: the important role of PI3K/Akt/mTOR in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer (Tokyo Japan) 25:392–401
Chang M (2012) Tamoxifen resistance in breast cancer. Biomol Ther (Seoul) 20(3):256–267
Ali S et al (2016) Molecular mechanisms and mode of tamoxifen resistance in breast cancer. Bioinformation 12(3):135–139
Sikora MJ et al (2014) Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res 74(5):1463–1474
de Herreros AG et al (2010) Snail family regulation and epithelial mesenchymal transitions in breast cancer progression. J Mammary Gland Biol Neoplasia 15(2):135–147
Martin TA et al (2005) Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol 12(6):488–496
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–428
Moody SE et al (2005) The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8(3):197–209
Elloul S et al (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103(8):1631–1643
Dubois-Marshall S et al (2011) Two possible mechanisms of epithelial to mesenchymal transition in invasive ductal breast cancer. Clin Exp Metastasis 28(8):811–818
Chen WJ et al (2010) Multidrug resistance in breast cancer cells during epithelial-mesenchymal transition is modulated by breast cancer resistant protein. Chin J Cancer 29(2):151–157
Kajita M, McClinic KN, Wade PA (2004) Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol 24(17):7559–7566
Proia TA et al (2011) Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 8(2):149–163
McCart Reed AE et al (2016) An epithelial to mesenchymal transition programme does not usually drive the phenotype of invasive lobular carcinomas. J Pathol 238(4):489–494
Morrogh M et al (2012) Cadherin-catenin complex dissociation in lobular neoplasia of the breast. Breast Cancer Res Treat 132(2):641–652
Jambal P et al (2013) Estrogen switches pure mucinous breast cancer to invasive lobular carcinoma with mucinous features. Breast Cancer Res Treat 137(2):431–448
Tasdemir N et al (2018) Comprehensive phenotypic characterization of human invasive lobular carcinoma cell lines in 2D and 3D cultures. Cancer Res 78(21):6209–6222
Meerbrey KL et al (2011) The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc Natl Acad Sci USA 108(9):3665–3670
Sikora MJ et al (2016) WNT4 mediates estrogen receptor signaling and endocrine resistance in invasive lobular carcinoma cell lines. Breast Cancer Res 18(1):92
Shaw FL et al (2012) A detailed mammosphere assay protocol for the quantification of breast stem cell activity. J Mammary Gland Biol Neoplasia 17(2):111–117
Harrell JC, Shroka TM, Jacobsen BM (2017) Estrogen induces c-Kit and an aggressive phenotype in a model of invasive lobular breast cancer. Oncogenesis 6(11):396
Scherbakov AM et al (2012) The relationships between snail1 and estrogen receptor signaling in breast cancer cells. J Cell Biochem 113(6):2147–2155
Wang M et al (2013) AIB1 cooperates with ERalpha to promote epithelial mesenchymal transition in breast cancer through SNAI1 activation. PLoS ONE 8(6):e65556
Ross-Innes CS et al (2012) Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481(7381):389–393
Christgen M, Derksen P (2015) Lobular breast cancer: molecular basis, mouse and cellular models. Breast Cancer Res 17:16
Zhou BP et al (2004) Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6(10):931–940
Lundgren K, Nordenskjold B, Landberg G, Hypoxia (2009) Snail and incomplete epithelial-mesenchymal transition in breast cancer. Br J Cancer 101(10):1769–1781
Vega S et al (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18(10):1131–1143
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Metzger Filho O et al (2015) Relative effectiveness of letrozole compared with tamoxifen for patients with lobular carcinoma in the BIG 1–98 trial. J Clin Oncol 33(25):2772–2779
Riggins RB et al (2008) ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res 68(21):8908–8917
Stires H et al (2018) Integrated molecular analysis of Tamoxifen-resistant invasive lobular breast cancer cells identifies MAPK and GRM/mGluR signaling as therapeutic vulnerabilities. Mol Cell Endocrinol 471:105–117
van Agthoven T et al (2015) Breast cancer anti-estrogen resistance 4 (BCAR4) drives proliferation of IPH-926 lobular carcinoma cells. PLoS ONE 10(8):e0136845
Sosa MS, Bragado P, Aguirre-Ghiso JA (2014) Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 14(9):611–622
Acknowledgements
The authors thank Donald DeFranco, Ph.D., Adrian Lee, Ph.D., and Carola Neumann, M.D., at the University of Pittsburgh, as well as Adam Feinberg, Ph.D. at Carnegie Melon University for intellectual discussion and guidance with this work, and Jennifer Xavier, Ph.D. at the University of Pittsburgh for her critical reading and editing of the manuscript. Additionally, the authors extend gratitude to the laboratories of Adrian V. Lee, Carola Neumann, Mei Zhang, Yi Huang and Steffi Oesterreich in the Women’s Cancer Research Center at the University of Pittsburgh for their support and suggestions.
Funding
Research funding for this project was provided in part by a Susan G. Komen Scholar award (SO; SAC160073) and the Breast Cancer Research Foundation (SO). EAB was supported by a Department of Pharmacology and Chemical Biology John S. Lazo Fellowship, and a National Institutes of Health Ruth L. Kirschstein award [1F31CA203055-01]. NT was supported by a Department of Defense Breakthrough Fellowship Award [BC160764]. MJS was supported by a Department of Defense Postdoctoral Fellowship [BC110619], and a National Institutes of Health Pathway to Independence award [K99 CA193734]. KL was supported by a National Institutes of Health Ruth L. Kirschstein award [1F30CA203154-01]. TFB has received research funding for EMT studies from an American Lung Association Award (LCD 257864), V Foundation Scholar Award, Sidney Kimmel Foundation (SKF-15-099) and a Doris Duke Charitable Foundation Clinical Scientist Award (2015097), a Pittsburgh LUNG SPORE CDA P50CA090440 and American Cancer Society Research Scholar Grant (132939-RSG-18-185-01 TBG). This project used UPMC Hillman Cancer Center Core Facilities, supported by and NCI award [P30CA047904].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Timothy F. Burns serves on an Advisory Board for AbbVie, Inc., and Dr. Oesterreich is a member of the External Scientific Advisory Board Panel of NSABP. The authors declare that they have no conflict of interest.
Research involving human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bossart, E.A., Tasdemir, N., Sikora, M.J. et al. SNAIL is induced by tamoxifen and leads to growth inhibition in invasive lobular breast carcinoma. Breast Cancer Res Treat 175, 327–337 (2019). https://doi.org/10.1007/s10549-019-05161-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05161-8