Abstract
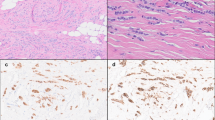
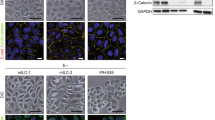
E-cadherin (E-CD) inactivation with loss of E-CD-mediated cell adhesion is the hallmark of lesions of the lobular phenotype. E-CD is typically absent by immunohistochemistry in both lobular carcinoma in situ (LCIS) and invasive lobular lesions, suggesting it occurs early in the neoplastic process. In laboratory models, downstream post-transcriptional modifiers such as TWIST and SNAIL contribute to the dissociation of the intracellular component of the cadherin–catenin complex (CCC), resulting in tumor progression and invasion. We hypothesized that complete CCC dissociation may play a role in lobular neoplasia progression. Here we explore the relationship between loss of E-CD and dissociation of the CCC in pure LCIS and LCIS associated with invasive cancer. Fresh-frozen tissues were obtained from 36 patients undergoing mastectomy for pure LCIS (n = 11), LCIS with ILC (n = 18) or LCIS with IDC (n = 7). Individual lesions were subject to laser-capture microdissection and gene-expression analysis (Affymetrix HG-U133A 2.0). Immunohistochemistry for ER,PR,HER2, E-CD,N-CD,α-,β-, and phosphoβ-catenin, TWIST, and SNAIL were evaluated in normal, in situ, and invasive components from matched formalin-fixed paraffin-embedded samples (n = 36). CCC-dissociation was defined as negative membranous E-CD, α- and β-catenin expression. E-CD was negative in all LCIS and ILC lesions, and positive in all normal and IDC lesions. Membranous α and β-catenin expressions decreased with the transition from LCIS to ILC (pure LCIS 82%; LCIS w/ILC 28%; ILC 0%), while TWIST expression increased (pure LCIS low; LCIS w/ILC moderate; ILC high). Gene expression paralleled IHC-staining patterns with a stepwise downregulation of E-CD, α and β-catenins from normal to LCIS to invasive lesions, and increasing expression of TWIST from normal to LCIS to ILC. Loss of E-CD expression is an early event in lobular neoplasia. Decreasing membranous catenin expression in tandem with increasing levels of TWIST across the spectrum of lobular lesions suggests that CCC dissociation is a progressive process.






Similar content being viewed by others
References
Hwang ES, Nyante SJ, Chen YY, Moore D, DeVries S, Korkola JE, Esserman LJ, Waldman FM (2004) Clonality of lobular carcinoma in situ and synchronous invasive lobular carcinoma. Cancer 100:2562–2572
Aulmann S, Penzel R, Longerich T, Funke B, Schirmacher P, Sinn HP (2008) Clonality of lobular carcinoma in situ (LCIS) and metachronous invasive breast cancer. Breast Cancer Res Treat 107:331–335
Morandi L, Marucci G, Foschini MP, Cattani MG, Pession A, Riva C, Eusebi V (2006) Genetic similarities and differences between lobular in situ neoplasia (LN) and invasive lobular carcinoma of the breast. Virchows Arch 449:14–23
Mastracci TL, Boulos FI, Andrulis IL, Lam WL (2007) Genomics and premalignant breast lesions: clues to the development and progression of lobular breast cancer. Breast Cancer Res 9:215
Jiang WG, Mansel RE (2000) E-cadherin complex and its abnormalities in human breast cancer. Surg Oncol 9:151–171
Klaus A, Birchmeier W (2008) Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8:387–398
Gould Rothberg BE, Bracken MB (2006) E-cadherin immunohistochemical expression as a prognostic factor in infiltrating ductal carcinoma of the breast: a systematic review and meta-analysis. Breast Cancer Res Treat 100:139–148
Nollet F, Berx G, van Roy F (1999) The role of the E-cadherin/catenin adhesion complex in the development and progression of cancer. Mol Cell Biol Res Commun 2:77–85
Baranwal S, Alahari SK (2009) Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun 384:6–11
Yang SZ, Kohno N, Yokoyama A, Kondo K, Hamada H, Hiwada K (2001) Decreased E-cadherin augments beta-catenin nuclear localization: studies in breast cancer cell lines. Int J Oncol 18:541–548
Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR (2004) E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 23:1739–1748
Berx G, Cleton-Jansen AM, Nollet F, de Leeuw WJ, van de Vijver M, Cornelisse C, van Roy F (1995) E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J 14:6107–6115
Berx G, Cleton-Jansen AM, Strumane K, de Leeuw WJ, Nollet F, van Roy F, Cornelisse C (1996) E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene 13:1919–1925
Masciari S, Larsson N, Senz J et al (2007) Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet 44:726–731
Rahman N, Stone JG, Coleman G, Gusterson B, Seal S, Marossy A, Lakhani SR, Ward A, Nash A, McKinna A, A’Hern R, Stratton MR, Houlston RS (2000) Lobular carcinoma in situ of the breast is not caused by constitutional mutations in the E-cadherin gene. Br J Cancer 82:568–570
Rieger-Christ KM, Pezza JA, Dugan JM, Braasch JW, Hughes KS, Summerhayes IC (2001) Disparate E-cadherin mutations in LCIS and associated invasive breast carcinomas. Mol Pathol 54:91–97
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939
Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, Reich R, Davidson B (2005) Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer 103:1631–1643
Come C, Magnino F, Bibeau F, De Santa PB, Becker KF, Theillet C, Savagner P (2006) Snail and slug play distinct roles during breast carcinoma progression. Clin Cancer Res 12:5395–5402
Morrogh M, Olvera N, Bogomolniy F, Borgen PI, King TA (2007) Tissue preparation for laser capture microdissection and RNA extraction from fresh frozen breast tissue. Biotechniques 43:41–42
Smyth GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3:3
Wolff AC, Hammond ME, Schwartz JN et al (2007) American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 131:18–43
Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO (2007) High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol 31:417–426
Simpson PT, Reis-Filho JS, Gale T, Lakhani SR (2005) Molecular evolution of breast cancer. J Pathol 205:248–254
Abdel-Fatah TM, Powe DG, Hodi Z, Reis-Filho JS, Lee AH, Ellis IO (2008) Morphologic and molecular evolutionary pathways of low nuclear grade invasive breast cancers and their putative precursor lesions: further evidence to support the concept of low nuclear grade breast neoplasia family. Am J Surg Pathol 32:513–523
Wagner PL, Kitabayashi N, Chen YT, Shin SJ (2009) Clonal relationship between closely approximated low-grade ductal and lobular lesions in the breast: a molecular study of 10 cases. Am J Clin Pathol 132:871–876
Cleton-Jansen AM (2002) E-cadherin and loss of heterozygosity at chromosome 16 in breast carcinogenesis: different genetic pathways in ductal and lobular breast cancer? Breast Cancer Res 4:5–8
Droufakou S, Deshmane V, Roylance R, Hanby A, Tomlinson I, Hart IR (2001) Multiple ways of silencing E-cadherin gene expression in lobular carcinoma of the breast. Int J Cancer 92:404–408
Lombaerts M, van Wezel T, Philippo K, Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de Water B, Cornelisse CJ, Cleton-Jansen AM (2006) E-cadherin transcriptional downregulation by promoter methylation but not mutation is related to epithelial-to-mesenchymal transition in breast cancer cell lines. Br J Cancer 94:661–671
Zou D, Yoon HS, Perez D, Weeks RJ, Guilford P, Humar B (2009) Epigenetic silencing in non-neoplastic epithelia identifies E-cadherin (CDH1) as a target for chemoprevention of lobular neoplasia. J Pathol 218:265–272
Caldeira JR, Prando EC, Quevedo FC, Neto FA, Rainho CA, Rogatto SR (2006) CDH1 promoter hypermethylation and E-cadherin protein expression in infiltrating breast cancer. BMC Cancer 6:48
Vesuna F, van Diest P, Chen JH, Raman V (2008) Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem Biophys Res Commun 367:235–241
Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Kleer CG, Varambally S, Chinnaiyan AM (2008) Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene 27:7274–7284
Sarrio D, Moreno-Bueno G, Hardisson D, Sanchez-Estevez C, Guo M, Herman JG, Gamallo C, Esteller M, Palacios J (2003) Epigenetic and genetic alterations of APC and CDH1 genes in lobular breast cancer: relationships with abnormal E-cadherin and catenin expression and microsatellite instability. Int J Cancer 106:208–215
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, Nieto MA (2002) Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene 21:3241–3246
Da Silva L, Parry S, Reid L, Keith P, Waddell N, Kossai M, Clarke C, Lakhani SR, Simpson PT (2008) Aberrant expression of E-cadherin in lobular carcinomas of the breast. Am J Surg Pathol 32:773–783
De Leeuw WJ, Berx G, Vos CB, Peterse JL, Van de Vijver MJ, Litvinov S, Van Roy F, Cornelisse CJ, Cleton-Jansen AM (1997) Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol 183:404–411
Acs G, Lawton TJ, Rebbeck TR, LiVolsi VA, Zhang PJ (2001) Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol 115:85–98
Nurismah MI, Noriah O, Suryati MY, Sharifah NA (2008) E-cadherin expression correlates with histologic type but not tumour grade in invasive breast cancer. Asian Pac J Cancer Prev 9:699–702
Hashizume R, Koizumi H, Ihara A, Ohta T, Uchikoshi T (1996) Expression of beta-catenin in normal breast tissue and breast carcinoma: a comparative study with epithelial cadherin and alpha-catenin. Histopathology 29:139–146
Goyal A, Martin TA, Mansel RE, Jiang WG (2008) Real time PCR analyses of expression of E-cadherin, alpha-, beta- and gamma-catenin in human breast cancer for predicting clinical outcome. World J Surg Oncol 6:56
Acknowledgments
Funding for this study was provided by the Breast Cancer Alliance, the Geoffrey Beene Cancer Research Center, the Association of Women Surgeons, and the Society of Surgical Oncology.
Conflict of interest
The authors report no conflicts of interest. The authors have control of all primary data and agree to allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mary Morrogh and Victor P. Andrade contributed equally to this work and share first authorship on this paper.
Rights and permissions
About this article
Cite this article
Morrogh, M., Andrade, V.P., Giri, D. et al. Cadherin–catenin complex dissociation in lobular neoplasia of the breast. Breast Cancer Res Treat 132, 641–652 (2012). https://doi.org/10.1007/s10549-011-1860-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-011-1860-0