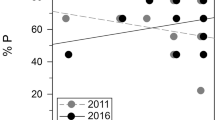
Abstract
Genetic diversity and reproductive mode can control whether an introduced species becomes invasive. Here we use genetic tools to compare the non-native, invasive Phragmites australis to its native conspecific, P. australis subsp. americanus, in wetlands of Utah and southern Idaho. We found striking differences in genetic structuring, population diversity, and mode of reproduction between the two lineages. Non-native P. australis exhibited substantially more genetic homogeneity among populations, greater local genet richness, greater genetic diversity among individuals, and smaller average clone size compared to the native lineage. These findings suggest that non-native P. australis relies more heavily on sexual reproduction and disperses pollen and/or seeds more widely than native P. australis. We also found no evidence of hybridization between the two lineages, nor did we find evidence of local extirpations of native by non-native P. australis based on historical collection sites we revisited. Given the ability of non-native P. australis to disperse widely by seeds, we recommend careful monitoring of critical wetland habitat to detect new non-native P. australis invasions and incorporating new practices into Phragmites management that limit sexual reproduction.






Similar content being viewed by others
References
Able KW, Hagan SM, Brown SA (2003) Mechanisms of marsh habitat alteration due to Phragmites: Response of young-of-the-year mummichog (Fundulus heteroclitus) to treatment for Phragmites removal. Est Coasts 26:484–494
Amsberry L, Baker MA, Ewanchuk PJ et al (2000) Clonal integration and the expansion of Phragmites australis. Ecol Appl 10:1110–1118
Baldwin AH, Kettenring KM, Whigham DF (2010) Seed banks of Phragmites australis-dominated brackish wetlands: relationships to seed viability, inundation, and land cover. Aquat Bot 93:163–169
Barrett SCH, Colautti RI, Eckert CG (2008) Plant reproductive systems and evolution during biological invasion. Mol Ecol 17:373–383
Bart D, Hartman JM (2000) Environmental determinants of Phragmites australis expansion in a New Jersey salt marsh: an experimental approach. Oikos 89:59–69
Bart D, Hartman JM (2002) Environmental constraints on early establishment of Phragmites australis in salt marshes. Wetlands 22:201–213
Bart D, Hartman JM (2003) The role of large rhizome dispersal and low salinity windows in the establishment of common reed, Phragmites australis, in salt marshes: New links to human activities. Est Coasts 26:436–443
Belzile F, Labbé J, LeBlanc M-C et al (2010) Seeds contribute strongly to the spread of the invasive genotype of the common reed (Phragmites australis). Biol Invasions 12:2243–2250
Benham JJ (2001) Genographer, version 1.6.0. Montana State University, Bozeman, Montana
Bossdorf O, Auge H, Lafuma L et al (2005) Phenotypic and genetic differentiation between native and introduced plant populations. Oecologia 144:1–11
Clevering OA, Lissner J (1999) Taxonomy, chromosome numbers, clonal diversity and population dynamics of Phragmites australis. Aquat Bot 64:185–208
Clevering OA, Brix H, Lukavska J (2001) Geographic variation in growth responses in Phragmites australis. Aquat Bot 69:89–108
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211
DeWalt SJ, Denslow JS, Ickes K (2004) Natural-enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecol 85:471–483
Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17:431–449
Duchesne P, Bernatchez L (2002) AFLPOP: a computer program for simulated and real population allocation, based on AFLP data. Mol Ecol Notes 2:380–383
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Nat Acad Sci 97:7043–7050
Ellstrand N, Schierenbeck K (2006) Hybridization as a stimulus for the evolution of invasiveness in plants? Euphyt 148:35–46
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 131:479–491
Facon B, Pointier J-P, Jarne P et al (2008) High genetic variance in life-history strategies within invasive populations by way of multiple introductions. Curr Biol 18:363–367
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–755
Genton BJ, Shykoff JA, Giraud T (2005) High genetic diversity in French invasive populations of common ragweed, Ambrosia artemisiifolia, as a result of multiple sources of introduction. Mol Ecol 14:4275–4285
Hansen DL, Lambertini C, Jampeetong A et al (2007) Clone-specific differences in Phragmites australis: Effects of ploidy level and geographic origin. Aquat Bot 86:269–279
Hardy OJ (2003) Estimation of pairwise relatedness between individuals and characterization of isolation-by-distance processes using dominant genetic markers. Mol Ecol 12:1577–1588
Hardy OJ, Vekemans X (2002) SPAGeDI: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol Ecol Notes 2:618–620
Hauswaldt JS, Glenn TC (2003) Microsatellite DNA loci from the Diamondback terrapin (Malaclemys terrapin). Mol Ecol Notes 3:174–176
Holdredge C, Bertness MD, Von Wettberg E et al (2010) Nutrient enrichment enhances hidden differences in phenotype to drive a cryptic plant invasion. Oikos 119:1776–1784
Hutchison DW, Templeton AR (1999) Correlation of pairwise genetic and geographic distance measures: inferring the relative influences of gene flow and drift on the distribution of genetic variability. Evolution 53:1898–1914
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Keller BEM (2000) Plant diversity in Lythrum, Phragmites, and Typha marshes, Massachusetts, U.S.A. Wetlands Ecol Manage 8:391–401
Kettenring KM, Whigham DF (2009) Seed viability and seed dormancy of non-native Phragmites australis in suburbanized and forested watersheds of the Chesapeake Bay, USA. Aquat Bot 91:199–204
Kettenring KM, McCormick MK, Baron HM et al (2010) Phragmites australis (common reed) invasion in the Rhode River subestuary of the Chesapeake Bay: disentangling the effects of foliar nutrients, genetic diversity, patch size, and seed viability. Est Coasts 33:118–126
Kettenring KM, McCormick MK, Baron HM et al (2011) Mechanisms of Phragmites australis invasion: feedbacks among genetic diversity, nutrients, and sexual reproduction. J Appl Ecol 48:1305–1313
Kirk H, Paul J, Straka J et al (2011) Long-distance dispersal and high genetic diversity are implicated in the invasive spread of the common reed, Phragmites australis (Poaceae), in northeastern North America. Am J Bot 98:1180–1190
Koppitz H, Kühl H (2000) To the importance of genetic diversity of Phragmites australis in the development of reed stands. Wetlands Ecol Manage 8:403–414
Kulmatiski A, Beard KH, Meyerson LA et al (2011) Nonnative Phragmites australis invasion into Utah wetlands. West N Am Nat 70:541–552
Lavergne S, Molofsky J (2007) Increased genetic variation and evolutionary potential drive the success of an invasive grass. Proc Nat Acad Sci 104:3883–3888
League M, Colbert E, Seliskar D et al (2006) Rhizome growth dynamics of native and exotic haplotypes of Phragmites australis (Common reed). Est Coasts 29:269–276
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Marks M, Lapin B, Randall J (1994) Phragmites australis (Phragmites communis): threats, management, and monitoring. Nat Areas J 14:285–294
McCormick MK, Kettenring KM, Baron HM et al (2010a) Extent and reproductive mechanisms of Phragmites australis spread in brackish wetlands in Chesapeake Bay, Maryland (USA). Wetlands 30:67–74
McCormick MK, Kettenring KM, Baron HM et al (2010b) Spread of invasive Phragmites australis in estuaries with differing degrees of development: genetic patterns, Allee effects and interpretation. J Ecol 98:1369–1378
Meirmans PG, van Tienderen PH (2004) GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Mol Ecol Notes 4:792–794
Meyerson LA, Chambers RM, Vogt KA (1999) The effects of Phragmites removal on nutrient pools in a freshwater tidal marsh ecosystem. Biol Invasions 1:129–136
Meyerson LA, Lambert AM, Saltonstall K (2010a) A tale of three lineages: expansion of common reed (Phragmites australis) in the U.S. Southwest and Gulf Coast. Inv Pl Sci Mngt 3:515–520
Meyerson LA, Viola DV, Brown RN (2010b) Hybridization of invasive Phragmites australis with a native subspecies in North America. Biol Invasions 12:103–111
Minchinton TE, Bertness MD (2003) Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecol Appl 13:1400–1416
Minchinton TE, Simpson JC, Bertness MD (2006) Mechanisms of exclusion of native coastal marsh plants by an invasive grass. J Ecol 94:342–354
Mock KE, Brim-Box JC, Miller MP et al (2004) Genetic diversity and divergence among freshwater mussel (Anodonta) populations in the Bonneville Basin of Utah. Mol Ecol 13:1085–1098
Mozdzer TJ, Zieman JC (2010) Ecophysiological differences between genetic lineages facilitate the invasion of non-native Phragmites australis in North American Atlantic coast wetlands. J Ecol 98:451–458
Mozdzer T, Zieman J, McGlathery K (2010) Nitrogen uptake by native and invasive temperate coastal macrophytes: Importance of dissolved organic nitrogen. Est Coasts 33:784–797
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Paetkau D (2004) The optimal number of markers in genetic capture-mark-recapture studies. J Wildl Manage 68:449–452
Paul J, Vachon N, Garroway C et al (2010) Molecular data provide strong evidence of natural hybridization between native and introduced lineages of Phragmites australis in North America. Biol Invasions 12:2967–2973
Peakall R, Smouse P (2006) GenAlEx 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Raicu P, Staicu S, Stoian V et al (1972) The Phragmites communis Trin. chromosome complement in the Danube Delta. Hydrobiol 39:83–89
Rejmánek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecol 77:1655–1661
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Saltonstall K (2002) Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proc Nat Acad Sci 99:2445–2449
Saltonstall K (2003a) Genetic variation among North American populations of Phragmites australis: implications for management. Est Coasts 26:444–451
Saltonstall K (2003b) Microsatellite variation within and among North American lineages of Phragmites australis. Mol Ecol 12:1689–1702
Saltonstall K (2003c) A rapid method for identifying the origin of North American Phragmites populations using RFLP analysis. Wetlands 23:1043–1047
Saltonstall K (2011) Remnant native Phragmites australis maintains genetic diversity despite multiple threats. Conserv Genet 12:1027–1033
Saltonstall K, Stevenson JC (2007) The effect of nutrients on seedling growth of native and introduced Phragmites australis. Aquat Bot 86:331–336
Saltonstall K, Peterson PM, Soreng RJ (2004) Recognition of Phragmites australis subsp. americanus (Poaceae: Arundinoideae) in North America: evidence from morphological and genetic analyses. SIDA 21:683–692
Saltonstall K, Glennon K, Burnett A et al (2007) Comparison of morphological variation indicative of ploidy level in Phragmites australis (Poaceae) from eastern North America. Rhodora 109:415–429
Saltonstall K, Lambert A, Meyerson LA (2010) Genetics and reproduction of common (Phragmites australis) and giant reed (Arundo donax). Inv Pl Sci Mngt 3:495–505
Silvertown J (2008) The evolutionary maintenance of sexual reproduction: evidence from the ecological distribution of asexual reproduction in clonal plants. Int J Pl Sci 169:157–168
Swearingen J, Saltonstall K (2010) Phragmites field guide: distinguishing native and exotic forms of common reed (Phragmites australis) in the United States
Talley TS, Levin LA (2001) Modification of sediments and macrofauna by an invasive marsh plant. Biol Invasions 3:51–68
Vasquez EA, Glenn EP, Brown JJ et al (2005) Salt tolerance underlies the cryptic invasion of North American salt marshes by an introduced haplotype of the common reed Phragmites australis (Poaceae). Mar Ecol Prog Ser 298:1–8
Vos P, Hogers R, Bleeker M et al (1995) AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res 23:4407–4414
Whitney KD, Gabler CA (2008) Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Div Dist 14:569–580
Windham L, Ehrenfeld JG (2003) Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecol Appl 13:883–897
Wright S (1946) Isolation by distance under diverse systems of mating. Genetics 31:39–59
Acknowledgments
We thank Jer Pin Chong for assistance with the molecular analysis, and Jared Baker and Mike Taylor for assistance in the field. Funding was provided by the Intermountain West Joint Venture, the US Fish and Wildlife Service, and the Utah Wetlands Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kettenring, K.M., Mock, K.E. Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biol Invasions 14, 2489–2504 (2012). https://doi.org/10.1007/s10530-012-0246-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-012-0246-5