Abstract
Objectives
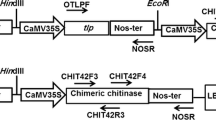
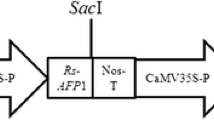
Sclerotinia stem rot (SSR) caused by Sclerotinia sclerotiorum is one of the major fungal diseases of canola. To develop resistance against this fungal disease, the chit42 from Trichoderma atroviride with chitin-binding domain and polygalacturonase-inhibiting protein 2 (PG1P2) of Phaseolus vulgaris were co-expressed in canola via Agrobacterium-mediated transformation.
Results
Stable integration and expression of transgenes in T0 and T2 plants was confirmed by PCR, Southern blot and RT-PCR analyses. Chitinase activity and PGIP2 inhibition were detected by colorimetric and agarose diffusion assay in transgenic lines but not in untransformed plants. The crude proteins from single copy transformant leaves having high chitinase and PGIP2 activity (T16, T8 and T3), showed up to 44 % inhibition of S. sclerotiorum hyphal growth. The homozygous T2 plants, showing inheritance in Mendelian fashion (3:1), were further evaluated under greenhouse conditions for resistance to S. sclerotiorum. Intact plants contaminated with mycelia showed resistance through delayed onset of the disease and restricted size and expansion of lesions as compared to wild type plants.
Conclusions
Combined expression of chimeric chit42 and pgip2 in Brassica napus L. provide subsequent protection against SSR disease and can be helpful in increasing the canola production in Iran.








Similar content being viewed by others
References
Aghajani MA, Safaie N, Alizadeh A (2013) Yield loss assessment of sclerotinia stem rot of canola in Iran. J Crop Prot 2:229–240
Akhgari AB, Motallebi M, Zamani MR (2012) Bean polygalacturonase-inhibiting protein expressed in transgenic Brassica napus inhibits polygalacturonase from its fungal pathogen Rhizoctonia solani. Plant Protect Sci 1:1–9
Benedetti M, Leggio C, Federici L, De Lorenzo G, Pavel NV, Cervone F (2011) Structural resolution of the complex between a fungal polygalacturonase and a plant polygalacturonase-inhibiting protein by small-angle X-ray scattering. Plant Physiol 157:599–607
Casasoli M, Federici L, Spinelli F, Di Matteo A, Vella N, Scaloni F, Fernandez-Recio J, Cervone F, De Lorenzo G (2009) Integration of evolutionary and desolvation energy analysis identifies functional sites in a plant immunity protein. Proc Natl Acad Sci USA 106:7666–7671
Ceasar SA, Ignacimuthu S (2012) Genetic engineering of crop plants for fungal resistance: role of antifungal genes. Biotechnol Lett 34:995–1002
Chhikara S, Dhankher OP, Chaudhury D, Jaiwal PK (2012) Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell Tissue Organ Cult 108:83–89
Cho HS, Cao J, Ren JP, Earle ED (2001) Control of lepidopteron insect pests in transgenic Chinese cabbage (Brassica rapa ssp. pekinensis) transformed with a synthetic Bacillus thuringiensis cry1C gene. Plant Cell Rep 20:1–7
Cletus J, Vashisht D, Balasubramanian V, Sakthivel N (2013) Transgenic expression of plant chitinases to enhance disease resistance. Biotechnol Lett 35:1719–1732
Cogbill S, Faulcon T, Jones G, McDaniel M, Harmon G, Blackmon R, Young M (2010) Adventitious shoots regeneration from cotyledonary explants of rapid-cycling fast plants of Brasscia rapa L. Plant Cell Tissue Organ Cult 101:127–133
Das DK, Rahman A (2012) Expression of a rice chitinase gene enhances antifungal response in transgenic litchi (cv. Bedana). Plant Cell Tissue Organ Cult 109:315–325
Das B, Goswami L, Ray S, Ghosh S, Bhattacharyya S, Das S, Majumder AL (2006) Agrobacterium-mediated transformation of Brassica juncea with a cyanobacterial (Synechocystis PCC6803) delta-6 desaturase gene leads to production of gamma-linolenic acid. Plant Cell Tissue Organ Cult 86:219–231
Dong X, Ji R, Guo X, Foster SJ, Chen H, Dong C, Liu Y, Hu Q, Liu S (2008) Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape. Planta 228:331–340
Doyle JJ, Doyle JL (1991) Isolation of plant DNA from fresh tissue plant. Plant Mol Biol 9:340–342
Ferrari S, Sella L, Janni M, De Lorenzo G, Favaron F, D’Ovidio R (2012) Transgenic expression of polygalacturonase-inhibiting proteins in Arabidopsis and wheat increases resistance to the flower pathogen Fusarium graminearum. Plant Biol 14:31–38
Harighi MJ, Motallebi M, Zamani MR (2006) Antifungal activity of heterologous expressed chitinase 42 (Chit42) from Trichoderma atroviride PTCC5220. Iran J Biotech 4:95–103
Hegedus DD, Li R, Buchwaldt L, Parkin I, Whitwill S, Coutu C, Bekkaoui D, Rimmer SR (2008) Brassica napus possesses an expanded set of polygalacturonase inhibitor protein genes that are differentially regulated in response to Sclerotinia sclerotiorum infection, wounding and defense hormone treatment. Planta 228:241–253
Iqbal MM, Ali S, Iqbal J, Asif MA, Nazir F, Zafar Y, Ali GM (2012) Over expression of rice chitinase gene in transgenic peanut (Arachis hypogaea L.) improves resistance against leaf spot. Mol Biotechnol 50:129–136
Janni M, Sella L, Favaron F, Blechl AE, De Lorenzo G, D’Ovidio R (2008) The expression of a bean PGIP in transgenic wheat confers increased resistance to the fungal pathogen Bipolaris sorokiniana. Mol Plant Microb 21:171–177
Kahrizi D, Salmanian AH, Afshari A, Moieni A, Mousavi A (2007) Simultaneous substitution of Gly96 to Ala and Ala183 to Thr in 5-enolpyruvylshikimate-3-phosphate synthase gene of E. coli (K12) and transformation of rapeseed (Brassica napus L.) in order to make tolerance to glyphosate. Plant Cell Rep 26:95–104
Kojima M, Yoshikawa T, Ueda M, Nonomura T, Matsuda Y, Toyoda H, Miyatake K, Arai M, Fukamizo T (2005) Family 19 chitinase from Aeromonas sp. No. 10S-24: role of chitin-binding domain in the enzymatic activity. J Biochem 137:235–242
Liu T, Liu L, Jiang X, Hou J, Fu K, Zhou F, Chen J (2010) Agrobacterium-mediated transformation as a useful tool for the molecular genetics study of the phytopathogen Curvularia lunata. Eur J Plant Pathol 126:363–371
Liu H, Guo X, Naeem MS, Liu D, Xu L, Zhang W, Tang G, Zhou W (2011) Transgenic Brassica napus L. lines carrying a two gene construct demonstrate enhanced resistance against Plutella xylostella and Sclerotinia sclerotiorum. Plant Cell Tiss Organ Cult 106:143–151
Matroodi S, Motallebi M, Zamani MR, Moradyar M (2013) Designing a new chitinase with more chitin binding and antifungal activity. World J Microbiol Biotechnol 29:1517–1523
Mohammadzadeh R, Zamani MR, Motallebi M, Norouzi P, Jourabchi E, Benedetti M, Lorenzo GD (2012) Agrobacterium tumefaciens-mediated introduction of polygalacturonase inhibiting protein 2 gene (PvPGIP2) from Phaseolus vulgaris into sugar beet (Beta vulgaris L.). Aust J Crop Sci 6:1290–1297
Moloney MM, Walker JM, Sharma KK (1989) High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Rep 8:238–242
Solgi T, Moradyar M, Zamani MR, Motallebi M (2015) Transformation of canola by Chit33 gene towards Improving resistance to Sclerotinia sclerotiorum. Plant Protect Sci 51:6–12
Sweetingham MW, Cruickshank RH, Wong DH (1986) Pectic zymograms and taxonomy and pathogenicity of the Ceratobasidiaceae. Trans Brit Mycol Soc 86:305–311
Taylor RJ, Secor GA (1988) An improved diffusion assay for quantifying the polygalacturonase content of Erwinia culture filtrates. Phytopathology 78:1101–1103
Xian H, Li D, Li J, Zhang L (2012) Cloning and functional analysis of a novel chitinase gene Trchi1 from Trichothecium roseum. Biotechnol Lett 34:1921–1928
Zeilinger S, Galhaup C, Payer K, Woo SL, Mach RL, Fekete C, Lorito M, Kubicek CP (1999) Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianumwith its host. Fungal Genet Biol 26:131–140
Acknowledgments
We would like to acknowledge National Institute of Genetic Engineering and Biotechnology (Project No. 407-M) for providing financial for this work.
Supporting Information
Supplementary Table 1—The sequence of primers used in PCR and Southern blot analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ziaei, M., Motallebi, M., Zamani, M.R. et al. Co-expression of chimeric chitinase and a polygalacturonase-inhibiting protein in transgenic canola (Brassica napus) confers enhanced resistance to Sclerotinia sclerotiorum . Biotechnol Lett 38, 1021–1032 (2016). https://doi.org/10.1007/s10529-016-2058-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-016-2058-7