Abstract
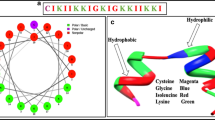
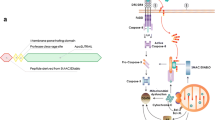
A novel activating peptide was designed and synthesized from V. cholerae hemagglutinine protease (HAP) mediated cleavage site of mouse PAR1. The peptide “PFISED” interacts with PAR1 in a new site which is different from its thrombin mediated conventional activation site and induced a series of new downstream signaling pathways. The peptide showed apoptosis in human and mouse breast (MCF-7 and EAC) and colon (HT29 and CT26) cancer cells where as in the same peptide concentration in normal human breast epithelial cells (MCF-10A), normal human fibroblast cells (MRC-5), normal mouse peritoneal macrophage cells and normal mouse breast and colon tissues did not show any effect. Treatment with this peptide enhanced the survival kinetics of EAC induced mice. The peptide mediated apoptosis was inhibited in presence of PAR1 inhibitor and was significantly reduced in si-PAR1 treated cells that indicate the activating peptide “PFISED” induced PAR1 mediated apoptosis of colon and breast cancer cells. This peptide induced over expression and activation of PAR1 and its downstream MAP kinase and NFκB signaling pathways. These signaling pathways enhanced the cellular ROS level to kill malignant cells. We report a novel pro-apoptotic peptide which can selectively kill malignant cells via its specific target receptor PAR1 which is over expressed in the malignant cells and can be used as a molecular target therapy for cancer treatment.







Similar content being viewed by others
References
Thundimadathil J (2012) Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. https://doi.org/10.1155/2012/967347
Jäkel CE, Meschenmoser K, Kim Y, Weiher H, Schmidt-Wolf IG (2012) Efficacy of a proapoptotic peptide towards cancer cells. In Vivo 26(3):419–426
Cragg GM, Kingston D, Newman DJ (2005) Anticancer agents from natural products. Brunner-Routledge Psychology Press, London, pp 186–205
Newman DJ, Cragg GM, Snader KM (2003) Natural products as a source of new drugs over the period 1981–2002. J Nat Prod 66:1022–1037. https://doi.org/10.1021/np030096l
Enb¨ack J, Laakkonen P (2007) Tumour-homing peptides: tools for targeting, imaging and destruction. Biochem Soc Trans 35(4):780–783. https://doi.org/10.1042/BST0350780
Aina OH, Sroka TC, Chen ML, Lam KS (2002) Therapeutic cancer targeting peptides. Biopolymers 66(3):184–199. https://doi.org/10.1002/bip.10257
Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M (2010) Synthetic therapeutic peptides: science and market. Drug Discov Today 15(1–2):40–56. https://doi.org/10.1016/j.drudis.2009.10.009
Qiu XQ, Wang H, Cai B, Wang LL, Yue ST (2007) Small antibody mimetics comprising two complementarity-determining regions and a framework region for tumor targeting. Nat Biotechnol 25(8):921–929. https://doi.org/10.1038/nbt1320
Allen TM (2002) Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer 2:750–763. https://doi.org/10.1038/nrc903
Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ (2006) Immunotoxin therapy of cancer. Nat Rev Cancer 6:559–565. https://doi.org/10.1038/nrc1891
Thorpe PE (2004) Vascular targeting agents as cancer therapeutics. Clin Cancer Res 10:415–427. https://doi.org/10.1158/1078-0432.CCR-0642-03
Mori T (2004) Cancer-specific ligands identified from screening of peptide-display libraries. Curr Pharm Des 10(19):2335–2343. https://doi.org/10.2174/1381612043383944
Reff ME, Hariharan K, Braslawsky G (2002) Future of monoclonal antibodies in the treatment of hematologic malignancies. Cancer Control 9(2):152–166. https://doi.org/10.1177/107327480200900207
Thayer AM (2011) Improving peptides. Chem Eng News 89(22):13–20. https://doi.org/10.1021/cen-v089n022.p013
Borghouts C, Kunz C, Groner B (2005) Current strategies for the development of peptide-based anti-cancer therapeutics. J Pept Sci 11(11):713–726. https://doi.org/10.1002/psc.717
Ray T, Chakrabarti MK, Pal A (2016) Hemagglutinin protease secreted by V. cholerae induced apoptosis in breast cancer cells by ROS mediated intrinsic pathway and regresses tumor growth in mice model. Apoptosis 21(2):143–154. https://doi.org/10.1007/s10495-015-1194-1
Han N, Jin K, He K, Cao J, Teng L (2011) Protease-activated receptors in cancer: a systematic review. Oncol Lett 2:599–608. https://doi.org/10.3892/ol.2011.291
Soh UJ, Dores MR, Chen B, Trejo J (2010) Signal transduction by protease-activated receptors. Br J Pharmacol 160(2):191–203. https://doi.org/10.1111/j.1476-5381.2010.00705.x
Turk B, Turk D, Turk V (2012) Protease signalling: the cutting edge. EMBO J 31(7):1630–1643. https://doi.org/10.1038/emboj.2012.42
Agarwal A, Covic L, Sevigny LM, Kaneider NC, Lazarides K, Azabdaftari G et al (2008) Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther 7:2746–2757. https://doi.org/10.1158/1535-7163.MCT-08-0177
Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A (2005) PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell 120:303–313. https://doi.org/10.1016/j.cell.2004.12.018
Pal S, Choudhuri T, Chattopadhyay S, Bhattacharya A, Datta GK, Das T et al (2001) Mechanisms of curcumin-induced apoptosis of Ehrlich’s ascites carcinoma cells. Biochem Biophys Res Commun 288:658–665. https://doi.org/10.1006/bbrc.2001.5823
Das T, Sa G, Chattopadhyay S, Ray PK (2002) Protein A-induced apoptosis of cancer cells is affected by soluble immune mediators. Cancer Immunol Immunother 51:376–380. https://doi.org/10.1007/s00262-002-0288-0
Gannon JV, Lane DP (1987) p63 and DNA polymerase a compete for the binding to SV40 T antigen. Nature 329:456–458
Rosner M, Hengstschlager M (2008) Cytoplasmic and nuclear distribution of the protein complexes mTORC1 and mTORC2: rapamycin triggers dephosphorylation and delocalization of the mTORC2 components rictor and sin1. Hum Mol Genet 17(19):2934–2948. https://doi.org/10.1093/hmg/ddn192
Ray T, Pal A (2016) PAR-1 mediated apoptosis of breast cancer cells by V.cholerae hemagglutinin protease. Apoptosis 21(5):609–620. https://doi.org/10.1007/s10495-016-1229-2
Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, Takeda N, Katayama K (2003) Broadly reactive and highly sensitive assay for norwalk-like viruses based on real-time Quantitative reverse transcription-PCR. J Clin Microbiol 41(4):1548–1557
Kumagai H, Mukaisho K, Sugihara H, Miwa K, Yamamoto G, Hattori T (2004) Thioproline inhibits development of esophageal adenocarcinoma induced by gastroduodenal reflux in rats. Carcinogenesis 25:723–727. https://doi.org/10.1093/carcin/bgh067
Ribble D, Goldstein NB, Norris DA, Shellman YG (2005) A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol 10:5–12. https://doi.org/10.1186/1472-6750-5-12
Ray T, Maity PC, Banerjee S, Deb S, Dasgupta AK, Sarkar S et al (2010) Vitamin C prevents cigarette smoke induced atherosclerosis in guinea pig model. J Atheroscler Thromb 17(8):817–827. https://doi.org/10.5551/jat.2881
Majumder B, Baraneedharan U, Thiyagarajan S, Radhakrishnan P, Narashisimhan H, Dhandapani M et al (2015) Predicting clinical response to anticancer drugs using an ex vivo platform that captures tumour heterogeneity. Nat Commun 6:6169. https://doi.org/10.1038/ncomms7169
Chattopadhyay S, Das T, Sa G, Ray PK (2002) Protein A-activated macrophages induce apoptosis in Ehrlich’s ascites carcinoma through a nitric oxide-dependent pathway. Apoptosis 7:49–57
Bhattacharyya A, Choudhuri T, Pal S, Chattopadhyay S, Datta GK, Sa G et al (2003) Apoptogenic effects of black tea on Ehrlich’s ascites carcinoma cell. Carcinogenesis 24(1):75–80. https://doi.org/10.1093/carcin/24.1.75
Roy T, Paul S, Baral RN, Chattopadhyay U, Biswas R (2007) Tumor associated release of interleukin-10 alters the prolactin receptor and down-regulates prolactin responsiveness of immature cortical thymocytes. J Neuroimmunol 186(1–2):112–120. https://doi.org/10.1016/j.jneuroim.2007.03.011
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Even-Ram S, Uziely B, Cohen P et al (1998) Thrombin receptor over expression in malignant and physiological invasion processes. Nat Med 4:909–914
Shi X, Gangadharan B, Brass L, Ruf W, Mueller B (2004) Protease activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res 2:395–402
Even-Ram SC, Maoz M, Pokroy E, Reich R, Katz BZ, Gutwein P, Altevogt P, Bar-Shavit R (2001) Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the αvβ5 integrin. J Biol Chem 276(914):10952–10962. https://doi.org/10.1074/jbc.M007027200
Kuliopulos A, Covic L, Seeley SK, Sheridan PJ, Helin J, Costello CE (1999) Plasmin desensitization of the PAR1 thrombin receptor: kinetics, sites of truncation, and implications for thrombolytic therapy. Biochemistry 38:4572–4585. https://doi.org/10.1021/bi9824792
Coughlin SR (1999) How the protease thrombin talks to cells. Proc Natl Acad Sci USA 96:11023–11027. https://doi.org/10.1073/pnas.96.20.11023
Chalmers CJ, Balmanno K, Hadfield K, Ley R, Cook SJ (2003) Thrombin inhibits Bim (Bcl-2-interacting mediator of cell death) expression and prevents serum-withdrawal-induced apoptosis via protease-activated receptor 1. Biochem J 375:99–109. https://doi.org/10.1042/bj20030346
Flynn AN, Buret AG (2004) Proteinase-activated receptor 1 (PAR-1) and cell apoptosis. Apoptosis 6:729–737. https://doi.org/10.1023/B:APPT.0000045784.49886.96
Acknowledgements
We would like to thank Dr. Rathin Baral (CNCI, India) for providing EAC and CT26 cells. We are grateful to the NICED central facility for FACS and confocal microscopy. Thanks to Mr Souvik Roy, DBT IPLS, CU for helping in the ITC experiment. TR is supported by the Bio-Care Women Scientist scheme of DBT, DK is supported by CSIR and SM is supported by N-PDF Scheme of DST-SERB.
Funding
Funding was provided by DBT-India with Grant No. [ BT/PR19713/BIC/101/237/2016 ].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Animals and ethics statement
Mice were maintained as per the principles and guidelines of the ethical committee for animal care of National Institute of Cholera and Enteric Diseases (NICED). The experimental design of the present study was approved by Institutional Animal Ethics Committee (License No: PRO/136/December 2016-December 2019), NICED, Kolkata, India.
Rights and permissions
About this article
Cite this article
Ray, T., Kar, D., Pal, A. et al. Molecular targeting of breast and colon cancer cells by PAR1 mediated apoptosis through a novel pro-apoptotic peptide. Apoptosis 23, 679–694 (2018). https://doi.org/10.1007/s10495-018-1485-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1485-4