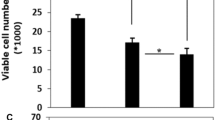
Abstract
Cathepsin D (CD), a ubiquitously expressed lysosomal aspartic protease, is upregulated in human breast carcinoma and many other tumor types. CD has been repeatedly reported to act as key mediator of apoptosis induced by various chemotherapeutics. However, there is still controversy over the role of enzymatic/proteolytic versus protein-protein interaction activities of CD in apoptotic signaling. The elucidation of molecular mechanism responsible for the effect of CD in the chemotherapy-induced cell death is crucial for development of an appropriate strategy to target this protease in cancer treatment. Therefore, the objective of this study was to investigate the molecular mechanism behind the CD-mediated regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cell death. For this purpose, MDA-MB-231 breast carcinoma cells with an increased level of wt CD (CD) or mutant enzymatically inactive CD (ΔCD) were subjected to TRAIL and the frequency of apoptosis was determined. Our results show that CD facilitates the TRAIL-induced apoptosis of MDA-MB-231 breast cancer cells in enzymatic activity-dependent manner. Moreover, the importance of endosomal/lysosomal acidification in this process was documented. Analysis of the potential substrates specifically cleaved by CD during the TRAIL-induced apoptosis confirmed caspase-8 and Bid proteins as the CD targets. Moreover, in search for protein regulators of apoptosis that can be cleaved by CD at physiologically relevant pH, we identified the Bcl-2 protein as a suitable candidate. The modulatory role of CD in cell response to TRAIL was also confirmed in another breast cancer cell line SKBR3. These experiments identified the CD enzymatic activity as a new factor affecting sensitivity of breast cancer cells to TRAIL.







Similar content being viewed by others
References
Benes P, Vetvicka V, Fusek M. Cathepsin D—many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28.
Margaryan NV, Kirschmann DA, Lipavsky A, Bailey CM, Hendrix MJC, Khalkhali-Ellis Z. New insights into cathepsin D in mammary tissue development and remodeling. Cancer Biol Ther. 2010;10:457–66.
Têtu B, Brisson J, Lapointe H, Wang CS, Bernard P, Blanchette C. Cathepsin D expression by cancer and stromal cells in breast cancer: an immunohistochemical study of 1348 cases. Breast Cancer Res Treat. 1999;55:137–47.
Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–78.
Garcia M, Derocq D, Pujol P, Rochefort H. Overexpression of transfected cathepsin D in transformed cells increases their malignant phenotype and metastatic potency. Oncogene. 1990;5:1809–14.
Glondu M, Liaudet-Coopman E, Derocq D, Platet N, Rochefort H, Garcia M. Down-regulation of cathepsin-D expression by antisense gene transfer inhibits tumor growth and experimental lung metastasis of human breast cancer cells. Oncogene. 2002;21:5127–34.
Knopfová L, Beneš P, Pekarčíková L, Hermanová M, Masařík M, Pernicová Z, et al. c-Myb regulates matrix metalloproteinases 1/9, and cathepsin D: implications for matrix-dependent breast cancer cell invasion and metastasis. Mol Cancer. 2012;11:15.
Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. EMBO J. 1996;15:3861–70.
Wu GS, Saftig P, Peters C, El-Deiry WS. Potential role for cathepsin D in p53-dependent tumor suppression and chemosensitivity. Oncogene. 1998;16:2177–83.
Emert-Sedlak L, Shangary S, Rabinovitz A, Miranda MB, Delach SM, Johnson DE. Involvement of cathepsin D in chemotherapy-induced cytochrome c release, caspase activation, and cell death. Mol Cancer Ther. 2005;4:733–42.
Démoz M, Castino R, Cesaro P, Baccino FM, Bonelli G, Isidoro C. Endosomal-lysosomal proteolysis mediates death signalling by TNFalpha, not by etoposide, in L929 fibrosarcoma cells: evidence for an active role of cathepsin D. Biol Chem. 2002;383:1237–48.
Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–63.
Kågedal K, Johansson U, Ollinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. Off Publ Fed Am Soc Exp Biol. 2001;15:1592–4.
Appelqvist H, Johansson A-C, Linderoth E, Johansson U, Antonsson B, Steinfeld R, et al. Lysosome-mediated apoptosis is associated with cathepsin D-specific processing of bid at Phe24, Trp48, and Phe183. Ann Clin Lab Sci. 2012;42:231–42.
Bidère N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, et al. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–11.
Carew JS, Espitia CM, Esquivel JA, Mahalingam D, Kelly KR, Reddy G, et al. Lucanthone is a novel inhibitor of autophagy that induces cathepsin D-mediated apoptosis. J Biol Chem. 2011;286:6602–13.
Castino R, Pace D, Démoz M, Gargiulo M, Ariatta C, Raiteri E, et al. Lysosomal proteases as potential targets for the induction of apoptotic cell death in human neuroblastomas. Int J Cancer J Int Cancer. 2002;97:775–9.
Tardy C, Tyynelä J, Hasilik A, Levade T, Andrieu-Abadie N. Stress-induced apoptosis is impaired in cells with a lysosomal targeting defect but is not affected in cells synthesizing a catalytically inactive cathepsin D. Cell Death Differ. 2003;10:1090–100.
Berchem G, Glondu M, Gleizes M, Brouillet J-P, Vignon F, Garcia M, et al. Cathepsin-D affects multiple tumor progression steps in vivo: proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951–5.
Hah Y-S, Noh HS, Ha JH, Ahn JS, Hahm JR, Cho HY, et al. Cathepsin D inhibits oxidative stress-induced cell death via activation of autophagy in cancer cells. Cancer Lett. 2012;323:208–14.
Marques C, Oliveira CSF, Alves S, Chaves SR, Coutinho OP, Côrte-Real M, et al. Acetate-induced apoptosis in colorectal carcinoma cells involves lysosomal membrane permeabilization and cathepsin D release. Cell Death Dis. 2013;4:e507.
Sagulenko V, Muth D, Sagulenko E, Paffhausen T, Schwab M, Westermann F. Cathepsin D protects human neuroblastoma cells from doxorubicin-induced cell death. Carcinogenesis. 2008;29:1869–77.
Beaujouin M, Baghdiguian S, Glondu-Lassis M, Berchem G, Liaudet-Coopman E. Overexpression of both catalytically active and -inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity. Oncogene. 2006;25:1967–73.
Laurent-Matha V, Maruani-Herrmann S, Prébois C, Beaujouin M, Glondu M, Noël A, et al. Catalytically inactive human cathepsin D triggers fibroblast invasive growth. J Cell Biol. 2005;168:489–99.
Linares CI, Ferrín G, Aguilar-Melero P, González-Rubio S, Rodríguez-Perálvarez M, Sánchez-Aragó M, et al. Sensitivity to anti-Fas is independent of increased cathepsin D activity and adrenodoxin reductase expression occurring in NOS-3 overexpressing HepG2 cells. Biochim Biophys Acta. 1853;2015:1182–94.
Masson O, Bach A-S, Derocq D, Prébois C, Laurent-Matha V, Pattingre S, et al. Pathophysiological functions of cathepsin D: targeting its catalytic activity versus its protein binding activity? Biochimie. 2010;92:1635–43.
Schestkowa O, Geisel D, Jacob R, Hasilik A. The catalytically inactive precursor of cathepsin D induces apoptosis in human fibroblasts and HeLa cells. J Cell Biochem. 2007;101:1558–66.
Oussama Achour NB. Cathepsin D activity and selectivity in the acidic conditions of a tumor microenvironment: utilization in the development of a novel cathepsin D substrate for simultaneous cancer diagnosis and therapy. Biochimie. 2013.
Hymowitz SG, Christinger HW, Fuh G, Ultsch M, O’Connell M, Kelley RF, et al. Triggering cell death: the crystal structure of Apo2L/TRAIL in a complex with death receptor 5. Mol Cell. 1999;4:563–71.
Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–20.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62.
Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–63.
Wang S. TRAIL: a sword for killing tumors. Curr Med Chem. 2010;17:3309–17.
van Dijk M, Halpin-McCormick A, Sessler T, Samali A, Szegezdi E. Resistance to TRAIL in non-transformed cells is due to multiple redundant pathways. Cell Death Dis. 2013;4:e702.
Werneburg NW, Guicciardi ME, Bronk SF, Kaufmann SH, Gores GJ. Tumor necrosis factor-related apoptosis-inducing ligand activates a lysosomal pathway of apoptosis that is regulated by Bcl-2 proteins. J Biol Chem. 2007;282:28960–70.
Spes A, Sobotič B, Turk V, Turk B. Cysteine cathepsins are not critical for TRAIL- and CD95-induced apoptosis in several human cancer cell lines. Biol Chem. 2012;393:1417–31.
Ohri SS, Vashishta A, Proctor M, Fusek M, Vetvicka V. The propeptide of cathepsin D increases proliferation, invasion and metastasis of breast cancer cells. Int J Oncol. 2008;32:491–8.
Ross DD, Joneckis CC, Ordóñez JV, Sisk AM, Wu RK, Hamburger AW, et al. Estimation of cell survival by flow cytometric quantification of fluorescein diacetate/propidium iodide viable cell number. Cancer Res. 1989;49:3776–82.
Repnik U, Česen MH, Turk B. The endolysosomal system in cell death and survival. Cold Spring Harb. Perspect. Biol. [Internet]. 2013 [cited 2013 Jun 14];5. Available from: http://cshperspectives.cshlp.org/content/5/1/a008755
Groth-Pedersen L, Jäättelä M. Combating apoptosis and multidrug resistant cancers by targeting lysosomes. Cancer Lett. 2010.
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87.
Horova V, Hradilova N, Jelinkova I, Koc M, Svadlenka J, Brazina J, et al. Inhibition of vacuolar ATPase attenuates the TRAIL-induced activation of caspase-8 and modulates the trafficking of TRAIL receptosomes. FEBS J. 2013;280:3436–50.
Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525.
Melzer IM, Fernández SBM, Bösser S, Lohrig K, Lewandrowski U, Wolters D, et al. The Apaf-1-binding protein Aven is cleaved by cathepsin D to unleash its anti-apoptotic potential. Cell Death Differ. 2012;19:1435–45.
Droga-Mazovec G, Bojic L, Petelin A, Ivanova S, Romih R, Repnik U, et al. Cysteine cathepsins trigger caspase-dependent cell death through cleavage of bid and antiapoptotic Bcl-2 homologues. J Biol Chem. 2008;283:19140–50.
Johansson A-C, Steen H, Ollinger K, Roberg K. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 2003;10:1253–9.
Dean RT. The aspartic proteinase inhibitor pepstatin, enters mouse peritoneal macrophages by adsorptive pinocytosis. Cell Biol Int Rep. 1983;7:405.
Yoshida H, Okamoto K, Iwamoto T, Sakai E, Kanaoka K, Hu J-P, et al. Pepstatin A, an aspartic proteinase inhibitor, suppresses RANKL-induced osteoclast differentiation. J Biochem (Tokyo). 2006;139:583–90.
Okada M, Irie S, Sawada M, Urae R, Urae A, Iwata N, et al. Pepstatin A induces extracellular acidification distinct from aspartic protease inhibition in microglial cell lines. Glia. 2003;43:167–74.
Conus S, Pop C, Snipas SJ, Salvesen GS, Simon H-U. Cathepsin D primes caspase-8 activation by multiple intra-chain proteolysis. J Biol Chem. 2012;287:21142–51.
Padrón-Barthe L, Courta J, Leprêtre C, Nagbou A, Torriglia A. Leukocyte elastase inhibitor, the precursor of L-DNase II, inhibits apoptosis by interfering with caspase-8 activation. Biochim Biophys Acta. 2008;1783:1755–66.
Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501.
Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2:318–25.
Nilsson C, Johansson U, Johansson A-C, Kågedal K, Ollinger K. Cytosolic acidification and lysosomal alkalinization during TNF-alpha induced apoptosis in U937 cells. Apoptosis Int J Program Cell Death. 2006;11:1149–59.
Park JR, Hockenbery DM. BCL-2, a novel regulator of apoptosis. J Cell Biochem. 1996;60:12–7.
Nencioni A, Wille L, Dal Bello G, Boy D, Cirmena G, Wesselborg S, et al. Cooperative cytotoxicity of proteasome inhibitors and tumor necrosis factor-related apoptosis-inducing ligand in chemoresistant Bcl-2-overexpressing cells. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:4259–65.
Acknowledgments
This work was funded by the European Regional Development Fund—projects FNUSA-ICRC (CZ.1.05/1.1.00/02.0123), CEB (CZ.1.07/2.3.00/20.0183), and by NT 13441-4/2012 (IGA, Ministry of Health of the Czech Republic), MEYS-NPS I-LO1413, MEYS-NPS II–LQ1605, and MH CZ–DRO (MMCI, 00209805) projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Jancekova, B., Ondrouskova, E., Knopfova, L. et al. Enzymatically active cathepsin D sensitizes breast carcinoma cells to TRAIL. Tumor Biol. 37, 10685–10696 (2016). https://doi.org/10.1007/s13277-016-4958-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-4958-5