Abstract
Prey are known to invest in costly antipredator behaviour when perceiving cues of dangerous, but not of relatively harmless predators. Whereas most studies investigate one type of antipredator behaviour, we studied several types (changes in oviposition, in escape and avoidance behaviour) in the spider mite Tetranychus evansi in response to cues from two predatory mites. The predator Phytoseiulus longipes is considered a dangerous predator for T. evansi, whereas Phytoseiulus macropilis has a low predation rate on this prey, thus is a much less dangerous predator. Spider mite females oviposited less on leaf disc halves with predator cues than on clean disc halves, independent of the predator species. On entire leaf discs, they laid fewer eggs in the presence of cues of the dangerous predator than on clean discs, but not in the presence of cues of the harmless predator. Furthermore, the spider mites escaped more often from discs with cues of the dangerous predator than from discs without predator cues, but they did not escape more from discs with cues of the harmless predator. The spider mites did not avoid plants with conspecifics and predators. We conclude that the spider mites displayed several different antipredator responses to the same predator species, and that some of these antipredator responses were stronger with cues of dangerous predators than with cues of harmless predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Predator–prey interactions are important for population dynamics, species composition (Paine 1966) and species distributions (Sih 1980; Kats and Dill 1998; Lima 1998). Predation results in removal of prey from a population and this can affect entire ecosystems (Taylor 1984). Moreover, predators also affect the behaviour of prey through their sheer presence or through cues associated with them. The consequences of these behavioural changes can be as important as predation, affecting prey reproductive success and dynamics, species composition and ecosystem processes (Schmitz 1998; Peacor and Werner 2001; Zanette et al. 2011). Prey can respond directly to attacks by predators by trying to escape, defending themselves and by counterattacking predators. They can also perceive the presence of predators before an actual attack through cues associated with the presence of predators to subsequently avoid an encounter. Many prey can perceive chemical cues that are associated with the presence of predators and subsequently change their behaviour to reduce predation risk (Chivers and Smith 1998; Kats and Dill 1998; Peckarsky et al. 2008; Paterson et al. 2013). In response to chemical cues, prey can seek refuge (Ives and Dobson 1987; Venzon et al. 2000; Faraji et al. 2001, 2002), avoid areas with predators (Gore 1966; Brown et al. 1995; Pallini et al. 1999; Magalhaes et al. 2002; Nomikou et al. 2003; Meng et al. 2006), increase their vigilance (Sweitzer and Berger 1992), and change their habitat (Lima and Dill 1990; Bolker et al. 2003).
Besides having the obvious benefit of reducing predation risk, antipredator behaviour usually carries costs because the behavioural changes go at the expense of the time and energy spent on other activities that are important for the fitness of prey individuals (Lima 1998; Pallini et al. 1998, 1999). These costs are responsible for the non-lethal effects of predators in prey (Lima 1998). For example, trying to escape from an area in which predator cues are perceived may result in more time and energy spent on locomotion and less time spent on feeding, which may ultimately result in slower growth, reduced investment in reproduction or delayed development (Spitze 1992; Barry 1994; Ylonen and Ronkainen 1994; Koskela and Ylonen 1995; Pallini et al. 1998; Zanette et al. 2011; Lasley-Rasher and Yen 2012). Increased vigilance of parents may also result in decreased reproduction and decreased survival of offspring (Zanette et al. 2011). Another example of costs of behavioural changes in species in which juveniles run a high risk of predation is the retention of eggs and the reduced investment in egg production in prey, which obviously results in lower oviposition rates (Montserrat et al. 2007; Hua et al. 2014). To reduce costs associated with antipredator behaviour, it is important that prey can discriminate among cues from dangerous and harmless predators (Sih 1987; Venzon et al. 2000; Persons et al. 2001), investing in costly antipredator behaviour only when perceiving cues of dangerous predators. To investigate this, we studied the response of spider mites to cues of dangerous and relatively harmless predators.
In spider mites, antipredator responses to predator cues can be manifested as the avoidance of areas and plants with predators (Grostal and Dicke 1999; Pallini et al. 1999; Magalhaes et al. 2002; Choh and Takabayashi 2007), changes in oviposition site (Grostal and Dicke 1999; Lemos et al. 2010) and moving away from the leaves on which they feed to avoid leaf-dwelling predators and subsequently entering diapause to survive a period of lack of food (Kroon et al. 2008). Furthermore, several studies have shown that spider mites that perceived predator cues had a reduced oviposition rate (Oku et al. 2004; Skaloudova et al. 2007; Choh et al. 2010). Whereas most of the papers cited above concentrate on one type of antipredator response, we study all these responses in one prey spider mite species exposed to cues of harmless and dangerous predators (except for diapause induction because our study animal does not enter diapause).
Spider mites colonize host plants and subsequently increase in numbers for several generations (Helle and Sabelis 1985a). Their most important enemies are predatory mites, which invade spider mite colonies and subsequently prey and reproduce there for several generations, eventually exterminating the prey population (Helle and Sabelis 1985b; Pels and Sabelis 1999). It is therefore important that predatory mites can reproduce on the spider mites present in the colony. The spider mite used here is Tetranychus evansi Baker & Pritchard (Acari: Tetranychidae), a pest of tomato plants in South America, Africa and Europe (Bolland and Vala 2000; Knapp et al. 2003; Saunyama and Knapp 2003; Escudero and Ferragut 2005; Wekesa et al. 2005). It stands out among the Tetranychidae because of its high population growth rate and large temperature range (Bonato 1999) and the production of dense web (Lemos et al. 2010), which is involved in reducing predation risk (Sabelis and Bakker 1992; Lemos et al. 2010) and competition (Sarmento et al. 2011b). Furthermore, the mite suppresses tomato plant defences to levels below that in uninfested plants (Sarmento et al. 2011a). Despite its importance as pest of tomato plants, not much is known about its response to predators (Lemos et al. 2010), which is important for developing biological control strategies.
Several studies have been devoted to finding an efficient biological control agent for T. evansi, mainly predatory mites from the family Phytoseiidae. However, many of these report negative results, also for the predatory mite Phytoseiulus macropilis Banks (Acari: Phytoseidae). This predator is successfully used for biological control of the spider mite Tetranychus urticae Koch, a species closely related to T. evansi (Watanabe et al. 1994; de Moraes et al. 2004; Oliveira et al. 2007, 2008, 2009). It can feed on all stages of prey, but eggs and young instars are more vulnerable (A. Janssen and F. Lemos, pers. obs.). However, P. macropilis has a low predation rate and cannot complete its life cycle when feeding on T. evansi and is not associated with it in the field (de Moraes and McMurtry 1985; Rosa et al. 2015). We therefore characterize it as a harmless predator for T. evansi.
In contrast, the closely related predatory mite Phytoseiulus longipes Evans (Acari: Phytoseidae) has a high population growth rate and high predation rate when feeding on T. evansi (de Moraes and McMurtry 1985). This predatory mite is also associated with Tetranychid populations in the field, it can develop and reproduce well when feeding on T. urticae or T. evansi (Ferrero et al. 2007; Furtado et al. 2007a), and is being considered as candidate for biological control of T. evansi populations on tomato (Furtado et al. 2007b; Silva et al. 2010; Ferrero et al. 2011). It can feed on all development stages of T. evansi, but eggs and young instars are most vulnerable. We therefore considered P. longipes as a dangerous predator for T. evansi.
We investigated the response of T. evansi to predator cues. The predators could not encounter the prey, but prey could perceive cues associated with predators. The objective of this study was to investigate whether T. evansi responded more strongly to cues of the dangerous predator P. longipes than to cues of the relatively harmless P. macropilis. We studied changes in oviposition behaviour of the spider mites to cues left behind by the predators, we assessed their escape behaviour in response to predator cues, and we studied their avoidance of plants with predator cues. In all cases, we expected that cues of dangerous predators would result in stronger changes of the behaviour than cues of harmless predators would.
Materials and methods
Rearing methods
Tomato plants (Solanum lycopersicum, variety Santa Clara I-5300) were grown in pots (2 L) using a commercial substrate, composed of vermiculite plus organic fertilizer. The plants were kept inside a greenhouse and fertilized with NPK (4-14-8) and superphosphate. The plants were watered according to their needs.
Tetranychus evansi was obtained in 2002 at the campus of the Federal University of Viçosa, Brazil, from naturally infested tomato plants of the same variety as above. Spider mites were cultured on tomato leaves with the petioles in a plastic tube with water to maintain leaf turgidity. The tubes were kept in plastic trays filled with detergent and water, which served to prevent mite escapes and invasion of other arthropods. A clean tomato leaf was added to the T. evansi culture every 2 days.
The predatory mite P. longipes was kindly provided by Professor Gilberto de Moraes (University of São Paulo, Brazil) in 2006. The predatory mite P. macropilis was found to spontaneously invade bean plants infested by T. urticae in a greenhouse on the campus mentioned above. Both P. longipes and P. macropilis were cultured in similar rearing units as the spider mites, but were supplied with leaves infested by spider mites. As explained above, P. macropilis cannot be reared on T. evansi, so it was fed T. urticae; the culture of P. longipes was fed T. evansi. Both predatory mite cultures were maintained under controlled conditions in a room (25 ± 2 °C, 80 ± 10 % relative humidity and 12 h light).
Oviposition in the presence of predator cues
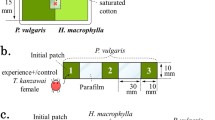
Leaf discs (Ø = 2.4 cm) were cut from tomato leaves, with the central vein in the middle of the discs. They were placed individually in a Petri dish (Ø = 4.0 cm) on top of a hydrophilic sponge soaked in water, which served to maintain the turgidity of the leaf disc. A thin line of hydrophilic cotton wool was placed on the vein, touching the water at both ends. Predatory mites cannot cross such humid cotton wool barriers (Pallini et al. 1998). Twenty-five adult female predators, either P. longipes or P. macropilis were placed on one of the disc halves; the other half remained clean. The predators and their eggs were carefully removed after 4 h. Hence, one disc half contained cues consisting of faeces, exuviae and chemical cues left by the predatory mites. These cues could be easily perceived by the spider mites because they were concentrated due to the high density of predators used. Subsequently, the cotton wool was removed and the vein was dried with tissue paper. One adult female spider mite, aged 12 days since egg, was released in the centre of each disc. Oviposition on each side of the disc was evaluated 24 h after release, and was analysed using a generalized linear model (GLM) with a quasi-Poisson error distribution. The effect of the cues of the two predator species on the distribution of eggs over disc halves was compared with a GLM with a quasi-binomial error distribution. Because spider mites are known to produce fewer eggs as a response to predator cues (Oku et al. 2004; Skaloudova et al. 2007; Choh et al. 2010), we also offered leaf discs entirely covered with predator cues or entirely clean. Discs of the same size as above either received 25 female predators for 4 h or were left clean. The same number of both species of predatory mites was used here as above, hence, the same amount of cues was present but on a twice as large a surface. The predators and their eggs were removed as above and one adult female spider mite was again released at the centre of each disc. The oviposition rate was measured as above. Twenty replicates were done per predator species. Oviposition rates were analysed using a GLM with Poisson error distribution with the presence of predator cues and predator species as factors.
Escapes from leaf discs with predator cues
In the above experiments, spider mites could only escape by entering the water surrounding the leaf discs, which they are somewhat reluctant to do. To evaluate escapes by T. evansi, we therefore prepared another type of arena, consisting of two discs (Ø = 2.5 cm) connected by a bridge (1.5 × 1.0 cm), cut as one piece from a single tomato leaflet. A thin line of hydrophilic cotton wool was placed on the connection of the bridge with the two discs to restrain mites on each disc. Ten females of T. evansi were released on both discs and 25 adult females of one of the two predator species were released on one of the discs 24 h later. Four hours after releasing the predators, the herbivores, predators and the cotton wool were removed from the discs and the arena was dried. Hence, these discs contained cues of prey, predators and cues associated with predation, in contrast to the previous experiments, in which only cues of predators were present. One female of T. evansi was released at the centre of the disc with T. evansi and predator cues, from where she could escape to the disc with only T. evansi cues. As control, one female of T. evansi was released on one of two connected discs, both only with T. evansi cues. The position of the female was evaluated after 48 h. Twenty replicates were done per treatment per predator species. The incidence of spider mites that had left the release disc after 48 h was analysed with a GLM with a binomial (P. longipes) and quasi-binomial (P. macropilis) error distribution.
Avoidance of plants with predator cues
The three highest leaves of six tomato plants with four completely developed leaves were infested with T. evansi, around 400 spider mites per plant, during 7 days. To prevent spider mites from moving from the upper leaves to the lowest leaf, a ring of glue was applied to the main stem of the plants immediately above the first leaf, isolating it from the rest of the plant (Pallini et al. 1999). Six days after infestation, the plants were allocated equidistantly in a hexagon (Ø = 80 cm) in a cage consisting of a frame (160 × 160 × 120 m) covered with thin screen and a tarpaulin bottom (160 × 160 × 15 cm) filled with soil. The pots containing the plants were buried and soil was added to the pots so that soil levels inside and outside the pots were equal (Pallini et al. 1998; Janssen 1999; see Nomikou et al. 2003 for a drawing of the set-up). The cage was situated outside, in an area shielded from extreme wind by a building on one side and a steep slope opposite, thus ensuring that the mites could not reach the plants through dispersal on air currents, but only by walking. To provide cues of predation risk, 100 adult females of P. longipes or P. macropilis were added to the three highest leaves with spider mites of three of the plants. Plants without predators were alternated with plants with predators in the hexagon. To control for any unforeseen directionality in the searching behaviour of the mites, care was taken that each plant position was occupied by plants without predators in half of the replicates, and by plants with predators in the other half (Janssen 1999). Hence, there were two configurations, one with plants with predators on positions 1, 3, and 5, and one with plants with predators on positions 2, 4, and 6.
Two hundred adult female spider mites were collected in a Petri dish, which was placed in the middle of the hexagon 24 h after the predators were added to the plants. The spider mites could disperse freely by walking over the soil to the plants. The stem of tomato plants contains high densities of trichomes, which impede movement of mites. Therefore, a wooden stick was inserted into the soil near the base, touching the lowest leaf, forming a bridge from the soil surface to the first leaf, giving the mites access to the leaf. Because of the glue barrier, the spider mites could not reach the leaves with predators, so they could only perceive volatile cues associated with predators. During the first 6 h and subsequently after 24 and 30 h, the mites that arrived on the lowest leaf of the plants were counted and removed.
To verify whether the set-up was suitable for assessing preference of T. evansi, we made use of the preference of this mite for plants with conspecifics over clean plants (Sarmento et al. 2011a). Three plants infested by T. evansi interspersed with three clean plants were placed in the same hexagon and spider mites were released at the center.
All experiments were replicated four times with four different sets of plants and different groups of mites. Square-root transformed numbers of mites recaptured on the plants were analysed using a linear mixed effects model (LME of the nlme package in R, R Development Core Team 2013). Replicates were used as random factor and the treatments of plants (with or without predators), time and the configuration of plants within the hexagon as factors.
Results
Oviposition in the presence of predator cues
Spider mites laid more eggs on the clean disc halves than on halves with cues of P. longipes (GLM: F1,38 = 7.66, p = 0.0086) or of P. macropilis (F1,34 = 4.54, p = 0.04) (Fig. 1a). When comparing the response of T. evansi to both predator species, there was no significant difference in oviposition behaviour (GLM: F1,36 < 0.001, p = 0.98; Fig. 1a). With cues of P. longipes, females produced on average 4.1 (SE = 0.57) eggs on the clean disc half and 1.65 (SE = 0.57) on the half with cues. With cues of P. macropilis, they produced 2.72 (SE = 0.57) eggs on the clean half and 1.11 (SE = 0.46) on the half with predator cues.
Mean (+SE, n = 20 females) oviposition of Tetranychus evansi after 24 h. a One half of each leaf disc had cues of predators whereas the other half did not. b Discs were either completely covered with cues of Phytoseiulus longipes or P. macropilis, or without such cues. Black bars indicate oviposition on discs (b) or disc halves (a) without predator cues, white bars that on discs (b) or disc halves (a) with predator cues. Asterisks indicate significant differences between treatment and control for each predator species separately; different letters indicate a significant difference between treatments with the different predator species, but for the same treatment, i.e. comparing the two white bars or the two black bars (GLM, p < 0.05)
When entire discs had cues of predators, T. evansi laid significantly fewer eggs on the disc with cues of P. longipes than on clean discs (GLM: Deviance = 10.05, df = 1,37, p = 0.0015; Fig. 1b). In contrast, the spider mites showed no significant difference in oviposition on the discs with or without P. macropilis cues (GLM: Deviance = 0.35, df = 1,33, p = 0.55; Fig. 1b). When comparing the response to both predators, T. evansi laid fewer eggs on discs with cues of P. longipes than with P. macropilis cues (GLM: Deviance = 12.5, df = 1,38, p = 0.0004; Fig. 1b). Oviposition on clean discs did not differ (i.e. the controls of the experiments with P. longipes and P. macropilis cues, GLM: Deviance < 0.001, df = 1,38, p = 1; Fig. 1b).
Escapes from leaf discs with predator cues
Tetranychus evansi escaped significantly more from discs with cues of P. longipes to a connected disc without predator cues than from discs without predator cues (GLM: Deviance = 10.9, df = 1,36, p = 0.001; Fig. 2). This did not hold for spider mites experiencing cues of P. macropilis (GLM: Deviance = 0.15, df = 1,32, p = 0.70; Fig. 2). When comparing the escapes between experiments, the proportion of escapes from discs with cues of only prey only did not differ significantly (cf. dark bars of Fig. 2, GLM: Deviance = 2.81, df = 1,35, p = 0.093), neither did the numbers of escapes from discs with predator cues (cf. light bars of Fig. 2, Deviance = 1.56, df = 1,33, p = 0.21).
Proportion (+SE, n = 20 females) of Tetranychus evansi that escaped from the arena where they were released. Black bars indicate the proportion of mites that escaped from discs without predator cues, white bars indicate that from discs with predator cues (Phytoseiulus longipes or P. macropilis). Asterisks indicate significant differences between treatment and control for each predator species separately; different letters indicate a significant difference between treatments with the different predator species, but for the same treatment, i.e. comparing the two white bars or the two black bars (GLM, p < 0.05)
Avoidance of plants with predator cues
Spider mites were recaptured more often on plants infested with conspecifics than on clean plants (LME, Likelihood ratio = 9.87, df = 4, p = 0.0017; Fig. 3a) and there was a significant effect of time on the numbers of mites recaptured (LME, Likelihood ratio = 30.2, df = 4, p < 0.0001). These results show that the mites can discriminate between plants that received different treatments in the set-up used.
Cumulative number (±SE) of Tetranychus evansi recaptured per plant through time on plants. a Plants either contained T. evansi (empty circles) or were clean (filled circles); b plants either contained T. evansi (empty circles) or T. evansi plus the predatory mite Phytoseiulus longipes (filled circles); c plants either contained T. evansi (empty circles) or T. evansi plus the predatory mite P. macropilis (filled circles)
There was no significant difference in the numbers of T. evansi recaptured on plants with or without P. longipes or P. macropilis (Fig. 3c, LME, Likelihood ratio = 0.48, df = 5, p = 0.48; Fig. 3b, Likelihood ratio = 0.16, df = 6, p = 0.68, respectively). There was a significant effect of time (LME, Likelihood ratio = 7.06, df = 5, p = 0.0079, Fig. 3) and position (Likelihood ratio = 5.38, df = 5, p = 0.02) on the numbers of mites recaptured in the experiment with P. longipes cues. For unknown reasons, more spider mites were recaptured on all plants when plants with P. longipes were on positions 1, 3, and 5 than when plants with these predators were on positions 2, 4, and 6. In the experiments with P. macropilis, there was a significant effect of the interaction between time and configuration (LME, Likelihood ratio = 6.03, df = 5, p = 0.014) on the numbers of mites recaptured. This was caused by an increase in the numbers of spider mites recaptured on plants without predators after 30 h, but only in one of the two configurations (data not shown). The reason for this increase is unknown so far. Nevertheless, the overall numbers of spider mites recaptured on plants with or without P. macropilis did not differ significantly. Comparison of the total percentage of spider mites recaptured in the three release experiments showed that T. evansi was recaptured more often when it was offered plants infested with T. evansi and clean plants (51.1 %) than when it was offered plants infested with T. evansi and predatory mites (P. longipes 24.3 % and P. macropilis 33.0 %) (GLM, F2,10 = 13.2, p < 0.001, Fig. 3).
Discussion
Many prey evolved the capacity to detect cues associated with predators and change their behaviour in response to those cues, even in the absence of predators (Chivers and Smith 1998; Kats and Dill 1998). We show here that the spider mite T. evansi changes its behaviour in response to cues of dangerous and harmless predators. Moreover, we found that this spider mite shows various types of behavioural changes to cues of the same predator. We found partial support for the expectation that the spider mites would show a stronger response to cues of the dangerous predator than to cues of the harmless predator. Specifically, we assessed three responses that were studied separately in spider mites thus far, i.e. their oviposition behaviour, escape behaviour and avoidance. First, the spider mites showed changes in oviposition behaviour, laying eggs more often on leaf disc halves without predator cues than on halves with predator cues. Contrary to expectation, the response to cues of the harmless predator did not differ from that to cues of the dangerous predator (Fig. 1a). A possible explanation for this is that the spider mites could move from one leaf disc half to the other within a few minutes; hence, they did not loose much time or energy because of this antipredator behaviour. Possibly, it then pays to even respond to cues of relatively harmless predators.
Furthermore, the spider mites produced fewer eggs on leaf discs entirely covered with predator cues than on clean leaf discs. The response to cues of the dangerous predator was stronger than that to cues of the harmless predator (Fig. 1b), which can be explained by the costs involved in this antipredator behaviour; reduced oviposition results in reduced offspring production. This reduced oviposition might have been caused by prey retaining their eggs to avoid predation (Montserrat et al. 2007). Alternatively, the spider mites may have tried to escape from the leaf discs, thus spending less time feeding. Because feeding is strongly related to egg production in spider mites (Sabelis 1991), this will also have resulted in lower oviposition rates. Further experiments should be done to clarify whether the reduced oviposition rate was caused by egg retention, by reduced feeding, or by both (Montserrat et al. 2007).
Second, the spider mites escaped more frequently from leaf discs with cues of the dangerous predator than from clean discs (Fig. 2), but not from discs with cues of the harmless predator (Fig. 2), again showing that the response to cues of the dangerous predator was stronger that that to cues of the harmless predator. In this case, escaping to the leaf disc with predator cues would involve more time than walking from one leaf disc half to another, as above. The spider mites had to find the bridge connecting two leaf discs and had to cover a larger distance to arrive at the other leaf disc.
Third, spider mites did not avoid plants with predators: similar numbers were recaptured on plants with predators as on plants without predators (Fig. 3b, c). It could be argued that the predation risk of individual mites on a plant with hundreds of adult spider mites and numerous eggs and immatures is rather low, thus, not avoiding plants with predators would not increase predation risk very much. However, earlier experiments using the same experimental set-up showed that a closely related spider mite species did avoid going to plants with one species of predator, but not to plants with another predator species (Pallini et al. 1999). We recaptured more spider mites when they were offered plants infested with T. evansi and clean plants than when offered plants infested with T. evansi and predatory mites (cf. Figure 3a with 3b, c). Hence, in the presence of cues from predators, fewer spider mites were found back on any plant (including plants without predators). Possibly, the spider mites tried to move away from plants with predators and thus spent more time walking before reaching a plant, or they chose to disperse away from the entire experimental arena in response to cues associated with predators. If so, the spider mites did also show antipredator behaviour in this experiment. In any case, they did not respond differently to the two predator species.
The cues perceived by the spider mites differed among experiments. Whereas the cues present in the experiments with the single leaf discs originated from the predators (feces, exuviae), the experiment with the connected discs also involved cues associated with predation, such as remains of dead conspecifics and perhaps prey alarm pheromones (Brown et al. 1995; Janssen et al. 1997; Grostal and Dicke 1999; Chivers and Smith 1998). Predation also occurred in the release-recapture experiment, but in this experiment, the spider mites could only perceive volatile cues, associated with conspecifics and with predation. Experiments with closely related spider mites and predators have demonstrated the existence of such volatile cues (Janssen et al. 1997; Pallini et al. 1999).
The diet of predators is known to affect antipredator behaviour: prey show stronger antipredator behaviour when perceiving cues from predators that recently fed on the same prey species than when perceiving predators that fed on other prey species (Venzon et al. 2000; Persons et al. 2001; van Maanen et al. 2015). Here, the predatory mite P. longipes was reared with T. evansi as prey and P. macropilis was reared on T. urticae. It is possible that T. evansi showed a weaker change in oviposition behaviour to P. macropilis because it had previously fed on another prey. This does not hold for the experiments on escape and avoidance behaviour, where both predator species had been feeding on T. evansi for some time.
In conclusion, our results show that the prey changed their behaviour when perceiving predator cues, but their response to cues associated with dangerous predators was not always stronger than to cues associated with harmless predators. In general, it is expected that prey should discriminate between cues from dangerous and harmless predators when the costs of antipredator behaviour are high (Sih 1982; 1987; Venzon et al. 2000; Persons et al. 2001). The lack of difference in response to dangerous and harmless predators found in some experiments here may partly have been caused by the low costs involved in antipredator behaviour, such as moving for a short time and over short distances (Sih 1982). Although many studies have addressed costs and benefits of antipredator behaviour, they have often considered only one type of behaviour. Here we show that prey show various types of antipredator behaviour to cues associated with the same predator species. We suggest that the various options of antipredator behaviour open to prey should all be considered, and their relative costs and benefits should be assessed to predict the most adequate type of response under various circumstances.
References
Barry MJ (1994) The costs of crest induction for Diaphnia carinata. Oecologia 97:278–288
Bolker BM, Holyoak Krivan V, Rowe L, Schmitz O (2003) Connecting theoretical and empirical studies of trait-mediated interactions. Ecology 84:1101–1114
Bolland H, Vala F (2000) First record of the spider mite Tetranychus evansi (Acari: Tetranychidae) from Portugal. Entomol Ber 60:180
Bonato O (1999) The effect of temperature on life history parameters of Tetranychus evansi (Acari: Tetranychidae). Exp Appl Acarol 23:11–19
Brown GE, Chivers DP, Smith RJF (1995) Fathead minnows avoid conspecific and heterospecific alarm pheromones in the feces of northern pike. J Fish Biol 47:387–393
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5:338–352
Choh Y, Takabayashi J (2007) Predator avoidance in phytophagous mites: response to present danger depends on alternative host quality. Oecologia 151:262–267
Choh Y, Uefune M, Takabayashi J (2010) Predation related odours reduce oviposition in a herbivorous mite. Exp Appl Acarol 50:1–8
de Moraes GJ, McMurtry J (1985) Comparison of Tetranychus evansi and T. urticae (Acari: Tetranychidae) as prey for eight species of phytoseiid mites. Entomophaga 30:393–397
de Moraes GJ, McMurtry JA, Denmark HA, Campos CB (2004) A revised catalogue of the mite family Phytoseiidae. Zootaxa 1:434–494
Escudero LA, Ferragut F (2005) Life-history of predatory mites Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae) on four spider mite species as prey, with special reference to Tetranychus evansi (Acari : Tetranychidae). Biol Control 32:378–384
Faraji F, Janssen A, Sabelis MW (2001) Predatory mites avoid ovipositing near counterattacking prey. Exp Appl Acarol 25:613–623
Faraji F, Janssen A, Sabelis MW (2002) Oviposition patterns in a predatory mite reduce the risk of egg predation caused by prey. Ecol Entomol 27:660–664
Ferrero M, de Moraes GJ, Kreiter S, Tixier M-S, Knapp M (2007) Life tables of the predatory mite Phytoseiulus longipes feeding on Tetranychus evansi at four temperatures (Acari: Phytoseiidae, Tetranychidae). Exp Appl Acarol 41:45–53
Ferrero M, Atuahiva T, Calvo FJ, Tixier M-S, Kreiter S (2011) Biological control of Tetranychus evansi Baker & Pritchard and T. urticae Koch by Phytoseiulus longipes Evans, in tomato greenhouses in Spain [Acari: Tetranychidae, Phytoseiidae]. Biol Control 58:30–35
Furtado IP, de Moraes GJ, Kreiter S, Tixier M-S, Knapp M (2007a) Potential of a Brazilian population of the predatory mite Phytoseiulus longipes as a biological control agent of Tetranychus evansi (Acari: Phytoselidae: Tetranychidae). Biol Control 42:139–147
Furtado IP, Toledo S, de Moraes GJ, Kreiter S, Knapp M (2007b) Search for effective natural enemies of Tetranychus evansi (Acari: Tetranychidae) in Northwest Argentina. Exp Appl Acarol 43:121–127
Gore RH (1966) Observations on escape response in Nassarius vibex (Say) (Mollusca: Gastropoda). Bull Mar Sci 16:423–431
Grostal P, Dicke M (1999) Direct and indirect cues of predation risk influence behavior and reproduction of prey: a case for acarine interactions. Behav Ecol 10:422–427
Helle W, Sabelis MW (eds) (1985a) Spider mites: their biology, natural enemies and control, World Crop Pests, vol 1A. Elsevier, Amsterdam
Helle W, Sabelis MW (eds) (1985b) Spider mites: their biology, natural enemies and control, World Crop Pests, vol 1B. Elsevier, Amsterdam
Hua F, Sieving KE, Fletcher RJ, Wright CA (2014) Increased perception of predation risk to adults and offspring alters avian reproductive strategy and performance. Behav Ecol 25:509–519
Ives AR, Dobson AP (1987) Antipredator behavior and the population dynamics of simple predator prey systems. Am Nat 130:431–447
Janssen A (1999) Plants with spider mite prey attract more predatory mites than clean plants under greenhouse conditions. Entomol Exp Appl 90:191–198
Janssen A, Bruin J, Jacobs G, Schraag R, Sabelis MW (1997) Predators use volatiles to avoid prey patches with conspecifics. J Anim Ecol 66:223–232
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Knapp M, Wagener B, Navajas M (2003) Molecular discrimination between the spider mite Tetranychus evansi Baker & Pritchard, an important pest of tomatoes in southern Africa, and the closely related species T. urticae Koch (Acarina: Tetranychidae). Afr Entomol 11:300–304
Koskela E, Ylonen H (1995) Suppressed breeding in the field vole (Microtus agrestis) an adaptation to cyclically fluctuating predation risk. Behav Ecol 6:311–315
Kroon A, Veenendaal RL, Bruin J, Egas M, Sabelis MW (2008) “Sleeping with the enemy” predator-induced diapause in a mite. Naturwissenschaften 95:1195–1198
Lasley-Rasher RS, Yen J (2012) Predation risk suppresses mating success and offspring production in the coastal marine copepod, Eurytemora herdmani. Limnol Oceanogr 57:433–440
Lemos F, Sarmento RA, Pallini A, Dias CR, Sabelis MW, Janssen A (2010) Spider mite web mediates antipredator behaviour. Exp Appl Acarol 52:1–10
Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions: What are the ecological effects of antipredator decision making? Bioscience 48:25–34
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Magalhaes S, Janssen A, Hanna R, Sabelis MW (2002) Flexible antipredator behaviour in herbivorous mites through vertical migration in a plant. Oecologia 132:143–149
Meng RX, Janssen A, Nomikou M, Zhang QW, Sabelis MW (2006) Previous and present diets of mite predators affect antipredator behaviour of whitefly prey. Exp Appl Acarol 38:113–124
Montserrat M, Bas C, Magalhaes S, Sabelis MW, de Roos AM, Janssen A (2007) Predators induce egg retention in prey. Oecologia 150:699–705
Nomikou M, Janssen A, Sabelis MW (2003) Herbivore host plant selection: whitefly learns to avoid host plants that harbour predators of her offspring. Oecologia 136:484–488
Oku K, Yano S, Takafuji A (2004) Nonlethal indirect effects of a native predatory mite, Amblyseius womersleyi Schicha (Acari:Phytoseiidae), on the phytophagous mite Tetranychus kanzawai Kishida (Acari: Tetranychidae). J Ethol 22:109–112
Oliveira H, Fadini MAM, Venzon M, Rezende D, Rezende F, Pallini A (2009) Evaluation of the predatory mite Phytoseiulus macropilis (Acari: Phytoseiidae) as a biological control agent of the two-spotted spider mite on strawberry plants under greenhouse conditions. Exp Appl Acarol 47:275–283
Oliveira H, Fadini MAM, Venzon M, Rezende D, Rezende F, Pallini A (2008) Evaluation of the predatory mite Phytoseiulus macropilis Banks (Acari: Phytoseiidae) as a biological control agent of the two-spotted spider mite on strawberry plants under greenhouse conditions. Exp Appl Acarol 47:275–283
Oliveira H, Janssen A, Pallini A, Venzon M, Fadini M, Duarte V (2007) A phytoseiid predator from the tropics as potential biological control agent for the spider mite Tetranychus urticae Koch (Acari: Tetranychidae). Biol Control 42:105–109
Paine RT (1966) Food web complexity and species diversity. Am Nat 100:10
Pallini A, Janssen A, Sabelis MW (1998) Predators induce interspecific herbivore competition for food in refuge space. Ecol Lett 1:171–177
Pallini A, Janssen A, Sabelis MW (1999) Spider mites avoid plants with predators. Exp Appl Acarol 23:803–815
Paterson RA, Pritchard DW, Dick JTA, Alexander ME, Hatcher MJ, Dunn AM (2013) Predator cue studies reveal strong trait-mediated effects in communities despite variation in experimental designs. Anim Behav 86:1301–1313
Peacor SD, Werner EE (2001) The contribution of trait-mediated indirect effects to the net effects of a predator. Proc Natl A Sci India B 98:3904–3908
Peckarsky BL, Abrams PA, Bolnick DI, Dill LM, Grabowski JH, Luttbeg B, Orrock JL, Peacor SD, Preisser EL, Schmitz OJ, Trussell GC (2008) Revisiting the classics: considering nonconsumptive effects in textbook examples of predator–prey interactions. Ecology 89:2416–2425
Pels B, Sabelis MW (1999) Local dynamics, overexploitation and predator dispersal in an acarine predator–prey system. Oikos 86:573–583
Persons MH, Walker SE, Rypstra AL, Marshall SD (2001) Wolf spider predator avoidance tactics and survival in the presence of diet associated predator cues (Araneae: Lycosidae). Anim Behav 61:43–51
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Stat Pinheiro istical Computing, Vienna
Rosa AA, Gondim JRMGC, Fiaboe KKM, de Moraes GJ, Knapp M (2015) Predatory mites associated with Tetranychus evansi Baker & Pritchard (Acari: Tetranychidae) on native solanaceous plants of coastal Pernambuco State, Brazil. Neotrop Entomol 34(4):689–692
Sabelis MW (1991) Life-history evolution of spider mites. In: Schuster R, Murphy PW (eds) The Acari reproduction, development and life-history strategies. Chapman and Hall, London, pp 23–49
Sabelis MW, Bakker FM (1992) How predatory mites cope with the web of their Tetranychid prey—a functional view on dorsal chaetotaxy in the Phytoseiidae. Exp Appl Acarol 16:203–225
Sarmento RA, Lemos F, Bleeker PM, Schuurink RC, Pallini A, Oliveira MGA, Lima ER, Kant M, Sabelis MW, Janssen A (2011a) A herbivore that manipulates plant defence. Ecol Lett 14:229–236
Sarmento RA, Lemos F, Dias CR, Kikuchi WT, Rodrigues JCP, Pallini A, Sabelis MW, Janssen A (2011b) A herbivorous mite down-regulates plant defence and produces web to exclude competitors. PLoS ONE 6(1–7):e23757
Saunyama IGM, Knapp M (2003) Effect of pruning and trellising of tomatoes on red spider mite incidence and crop yield in Zimbabwe. Afr Crop Sci J 11:269–277
Schmitz OJ (1998) Direct and indirect effects of predation and predation risk in old field interaction webs. Am Nat 151:327–342
Sih A (1980) Optimal behavior: Can foragers balance two conflicting demands? Science 210:1041–1043
Sih A (1982) Foraging strategies and the avoidance of predation by an aquatic insect, Notonecta hoffmanni. Ecology 63:786–796
Sih A (1987) Prey refuges and predator–prey stability. Theor Popul Biol 31:1–12
Silva FR, de Moraes GJ, Gondim MGC Jr, Knapp M, Rouam SL, Paes JLA, Oliveira GM (2010) Efficiency of Phytoseiulus longipes Evans as a control agent of Tetranychus evansi Baker & Pritchard (Acari: Phytoseiidae: Tetranychidae) on screenhouse tomatoes. Neotrop Entomol 39:991–995
Skaloudova B, Zemek R, Krivan V (2007) The effect of predation risk on an acarine system. Anim Behav 74:813–821
Spitze K (1992) Predator-mediated plasticity of prey life history and morphology: Chaoborus americanus predation on Daphnia pulex. Am Nat 139:229–247
Sweitzer RA, Berger J (1992) Size-related effects of predation on habitat use and behavior of porcupines (Erethizon dorsatum). Ecology 73:867–875
Taylor RJ (1984) Predation. Chapman and Hall, London
Van Maanen R, Broufas G, de Jong P, Aguilar-Fenollosa E, Revynthi A, Sabelis MW, Janssen A (2015) Predators marked with chemical cues from one prey have increased attack success on another prey species. Ecol Entomol 40:62–68
Venzon M, Janssen A, Pallini A, Sabelis MW (2000) Diet of a polyphagous arthropod predator affects refuge seeking of its thrips prey. Anim Behav 60:369–375
Watanabe MA, de Moraes GJ, Gastaldo I Jr, Nicolella G (1994) Controle biológico do ácaro rajado com ácaros predadores fitoseídeos em culturas de pepino e morango. Sci Agric 51:75–81
Wekesa VW, Maniania NK, Knapp M, Boga HI (2005) Pathogenicity of Beauveria bassiana and Metarhizium anisopliae to the tobacco spider mite Tetranychus evansi. Exp Appl Acarol 36:41–50
Ylonen H, Ronkainen H (1994) Breeding suppression in the bank vole as antipredatory adaptation in a predictable environment. Evol Ecol 8:658–666
Zanette LY, White AF, Allen MC, Clinchy M (2011) Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334:1398–1401
Acknowledgments
We thank the anonymous reviewers and colleagues from the laboratory of acarology of the University of Viçosa for support and constructive comments. CRD and AMGB were supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), AJ received a scholarship from the Foundation for Research Support of the State of Minas Gerais (FAPEMIG) (CBB-30003/09). CWCF was supported by the National Council for Scientific and Technological Development (CNPq). RAS received a scholarship from CNPq, AP was supported by CNPq, FAPEMIG and CAPES.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dias, C.R., Bernardo, A.M.G., Mencalha, J. et al. Antipredator behaviours of a spider mite in response to cues of dangerous and harmless predators. Exp Appl Acarol 69, 263–276 (2016). https://doi.org/10.1007/s10493-016-0042-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0042-5