Abstract
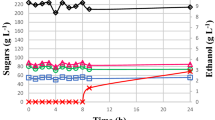
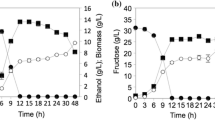
The yeast Dekkera bruxellensis is considered to be very well adapted to industrial environments, in Brazil, USA, Canada and European Countries, when different substrates are used in alcoholic fermentations. Our previous study described its fermentative profile with a sugarcane juice substrate. In this study, we have extended its physiological evaluation to fermentation situations by using sugarcane molasses as a substrate to replicate industrial working conditions. The results have confirmed the previous reports of the low capacity of D. bruxellensis cells to assimilate sucrose, which seems to be the main factor that can cause a bottleneck in its use as fermentative yeast. Furthermore, the cells of D. bruxellensis showed a tendency to deviate most of sugar available for biomass and organic acids (lactic and acetic) compared with Saccharomyces cerevisiae, when calculated on the basis of their respective yields. As well as this, the acetate production from molasses medium by both yeasts was in marked contrast with the previous data on sugarcane juice. Glycerol and ethanol production by D. bruxellensis cells achieved levels of 33 and 53 % of the S. cerevisiae, respectively. However, the ethanol yield was similar for both yeasts. It is worth noting that this yeast did not accumulate trehalose when the intracellular glycogen content was 30 % lower than in S. cerevisiae. The lack of trehalose did not affect yeast viability under fermentation conditions. Thus, the adaptive success of D. bruxellensis under industrial fermentation conditions seems to be unrelated to the production of these reserve carbohydrates.





Similar content being viewed by others
References
Bai FW, Anderson WA, Moo-Young M (2008) Ethanol fermentation technologies from sugar and starch feedstocks. Biotech Adv 26:89–105
Basílio ACM, Araújo PRL, Morais JOF, Silva-Filho EA, Morais MA Jr, Simões DA (2008) Detection and identification of wild yeast contaminants of the industrial fuel ethanol fermentation process. Curr Microbiol 56:322–326
Bassi AP, da Silva JC, Reis VR, Ceccato-Antonini SR (2013) Effects of single and combined cell treatments based on low pH and high concentrations of ethanol on the growth and fermentation of Dekkera bruxellensis and Saccharomyces cerevisiae. World J Microbiol Biotechnol 29:1661–1676
Basso LC, Amorim HV, Oliveira AJ, Lopes ML (2008) Yeast selection for fuel ethanol in Brazil. FEMS Yeast Res 8:1155–1163
Basso LC, Basso TO, Rocha SN (2011) Ethanol production in Brazil: the industrial process and its impact on yeast fermentation. In: Dos Santos Bernardes MA (ed) Biofuel production—recent developments and prospects. Intech, Zagreb, pp 85–100
Blomqvist J, Eberhard T, Schnürer J, Passoth V (2010) Fermentation characteristics of Dekkera bruxellensis strains. Appl Microbiol Biotechnol 87:1487–1497
Blomqvist J, Nogué VS, Gorwa-Grauslund M, Passoth V (2012) Physiological requirements for growth and competitiveness of Dekkera bruxellensis under oxygen-limited or anaerobic conditions. Yeast 29:265–274
Brendam C, Castro-Martínez C, Délia M-L, Ramón-Portugal F, Strehaianoet P (2008) Effect of temperature on Brettanomyces bruxellensis: metabolic and kinetic aspects. Can J Microbiol 54:11–18
Ciani M, Maccarelli F, Fatichenti F (2003) Growth and fermentation behaviour of Brettanomyces/Dekkera yeasts under different conditions of aerobiosis. World J Microbiol Biotechnol 19:419–422
Da Silva-Filho EA, Melo HF, Antunes DF, Dos Santos SKB, Resende MA, Simões DA, Morais MA Jr (2005) Isolation by genetics and physiological characteristics of a fuel ethanol fermentative Saccharomyces cerevisiae strain with potential for genetic manipulation. J Ind Microbiol Biotechnol 32:481–486
Dake MS, Jadhv JP, Patil NB (2010) Variations of two pools of glycogen and carbohydrate in Saccharomyces cerevisiae grown with various ethanol concentrations. J Ind Microbiol Biotechnol 37:701–706
De Barros Pita W, Leite FC, de Souza Liberal AT, Simões DA, de Morais MA (2011) The ability to use nitrate confers advantage to Dekkera bruxellensis over S. cerevisiae and can explain its adaptation to industrial fermentation processes. Antonie Van Leeuwenhoek 100:99–107
De Lucena RM, Elsztein C, Simões DA, De Morais Jr MA (2012) Participation of CWI, HOG and Calcineurin pathways in the tolerance of Saccharomyces cerevisiae to low pH by inorganic acid. J Appl Microbiol 113:629–640
De Souza Barros R, Dos Santos BM, De Souza RFR, Da Silva PQN, Lucena BL, Marcos Morais MA Jr (2012) The consequences of Lactobacillus vini and Dekkera bruxellensis as contaminants of the sugarcane-based ethanol fermentation. J Ind Microbiol Biotechnol 39(11):1645–1650. doi:10.1007/s10295-012-1167-0
De Souza Liberal AT, Basílio ACM, Resende AM, Brasileiro BTRV, Silva-Filho EA, Morais JOF, Simões DA, Morais MA Jr (2007) Identification of Dekkera bruxellensis as a major contaminant yeast in continuous fuel ethanol fermentation. J Appl Microbiol 102:538–547
De Virgilio C, Hottiger T, Dominguez J, Boller T, Wiemken A (1994) The role of trehalose synthesis for the acquisition of thermotolerance in yeast I. Genetic evidence that trehalose is a thermoprotectant. Eur J Biochem 219:179–186
Dorta C, Oliva-Neto P, De-Abreu-Neto MS, Nicolau-Junior N, Nagashima AI (2006) Synergism among lactic acid, sulfite, pH and ethanol in alcoholic fermentation of Saccharomyces cerevisiae (PE-2 and M-26). World J Microbiol Biotechnol 22:177–182
Elsztein C, Menezes JAS, Morais Junior MA (2008) Polyhexamethylene biguanide can eliminate contaminant yeasts from fuel-ethanol fermentation process. J Ind Microbiol Biotechnol 35:967–973
Francois J, Parrou JL, Graves T, Narendranath N, Power T (2001) Reserve carbohydrates metabolism in the yeast. J Inst Brew 113:263–271
Galafassi S, Toscano M, Vigentini I, Piškur J, Compagno C (2013) Osmotic stress response in the wine yeast Dekkera bruxellensis. Food Microbiol 36:316–319
Graves T, Narendranath N, Power R (2007) Development of a “stress model” fermentation system for fuel ethanol yeast strains. J Inst Brew 113:263–271
Jensen SL, Umiker NL, Arneborg N, Edwards CG (2009) Identification and characterization of Dekkera bruxellensis, Candida pararugosa and Pichia guilliermondii isolated from commercial red wines. Food Microbiol 26:915–921
Leite FCB, Basso TO, Pita WB, Gombert A, Simões D, Morais Junior MA (2012) Quantitative aerobic physiology of the yeast Dekkera bruxellensis, a major contaminant in bioethanol production plants. FEMS Yeast Res 13:34–43
Mansure JJ, Souza RC, Panek AD (1997) Trehalose metabolism in Saccharomyces cerevisiae during alcoholic fermentation. Biotechnol Lett 19:1201–1203
Martini S, Ricci M, Bartolini F, Bonechi C, Braconi D, Millucci L, Santucci A, Rossi C (2006) Metabolic response to exogenous ethanol in yeast: an in vivo NMR and mathematical modelling approach. Biophys Chem 120:135–142
Meneghin MC, Bassi AP, Codato CB, Reis VR, Ceccato-Antonini SR (2013) Fermentative and growth performances of Dekkera bruxellensis in different batch systems and the effect of initial low cell counts in co-cultures with Saccharomyces cerevisiae. Yeast 30:295–305
Parrou JL, Teste MA, Francois J (1997) Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiol 143:1891–1900
Passoth V, Blomqvist J, Schnürer J (2007) Dekkera bruxellensis and Lactobacillus vini form a stable ethanol-producing consortium in a commercial alcohol production process. Appl Environ Microbiol 73:4354–4356
Pataro C, Guerra JB, Gomes FCO, Neves MJ, Pimentel PF, Rosa CA (2002) Trehalose accumulation, invertase activity and physiological characteristics of yeasts isolated from 24 h fermentative cycles during the production of artisanal Brazilian cachaça. Braz J Microbiol 33:202–208
Pereira LF, Bassi APG, Avansini SH, Neto AGB, Brasileiro BTRV, Ceccato-antonini SR, De Morais MA (2012) The physiological characteristics of the yeast Dekkera bruxellensis in fully fermentative conditions with cell recycling and in mixed cultures with Saccharomyces cerevisiae. Antonie Leeuwenhoek 101:529–539
Renouf V, Falcou M, Miot-Sertier C, Perello MC, De Revel G, Lonvaud-Funel A (2006) Interactions between Brettanomyces bruxellensis and other yeast species during the initial stages of winemaking. J Appl Microbiol 100:1208–1219
Rocha-Leão MHM, Panek AD, Costa-Carvalho VLA (1984) Glycogen accumulation during growth of Saccharomyces cerevisiae: catabolite repression effects. IRCS Med Sci 12:411–412
Rozpedowska E, Hellborg L, Ishchuk OP, Orhan F, Galafassi S, Merico A, Woolfit M, Compagno C, Piškur J (2011) Parallel evolution of the make–accumulate–consume strategy in Saccharomyces and Dekkera yeasts. Nat Commun 2:302
Stambuk BU, Batista AS, De Araujo PS (2000) Kinetics of active sucrose transport in Saccharomyces cerevisiae. J Biosci Bioeng 89:212–214
Uscanga Aguilar MG, Delia ML, Strehaiano P (2003) Brettanomyces bruxellensis: effect of oxygen on growth and acetic acid production. Appl Microbiol Biotechnol 61(157–10):162
Van Dijken JP, Scheffers WA (1984) Studies on alcoholic fermentation in yeasts. In: Houwink EH, Van der Meer RR (eds) Innovations in biotechnology. Elsevier, Amsterdam, pp 497–506
Wijsman MR, van Dijken JP, van Kleeff BH, Scheffers WA (1984) Inhibition of fermentation and growth in batch cultures of the yeast Brettanomyces intermedius upon a shift from aerobic to anaerobic conditions (Custers effect). Antonie Van Leeuwenhoek 50:183–192
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, L.F., Lucatti, E., Basso, L.C. et al. The fermentation of sugarcane molasses by Dekkera bruxellensis and the mobilization of reserve carbohydrates. Antonie van Leeuwenhoek 105, 481–489 (2014). https://doi.org/10.1007/s10482-013-0100-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-013-0100-5