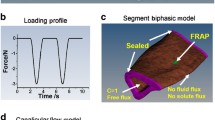
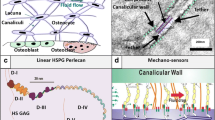
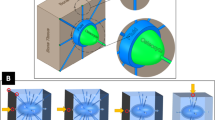
Abstract
Solute transport through the bone lacunar-canalicular system is essential for osteocyte viability and function, and it can be measured using fluorescence recovery after photobleaching (FRAP). The mathematical model developed here aims to analyze solute transport during FRAP in mechanically loaded bone. Combining both whole bone-level poroelasticity and cellular-level solute transport, we found that load-induced solute transport during FRAP is characterized by an exponential recovery rate, which is determined by the dimensionless Strouhal (St) number that characterizes the oscillation effects over the mean flows, and that significant transport occurs only for St values below a threshold, when the solute stroke displacement exceeds the distance between the source and sink (the canalicular length). This threshold mechanism explains the general flow behaviors such as increasing transport with increasing magnitude and decreasing frequency. Mechanical loading is predicted to enhance transport of all tracers relative to diffusion, with the greatest enhancement for medium-sized tracers and less enhancement for small and large tracers. This study provides guidelines for future FRAP experiments, based on which the model can be used to quantify bone permeability, solute–matrix interaction, and flow velocities. These studies should provide insights into bone adaptation and metabolism, and help to treat various bone diseases and conditions.









Similar content being viewed by others
References
Beno T., Yoon Y. J., Cowin S. C., Fritton S. P. (2006) Estimation of bone permeability using accurate microstructural measurements. J. Biomech. 39:2378–2387, doi:10.1016/j.jbiomech.2005.08.005
Bentolila V., Boyce T. M., Fyhrie D. P., Drumb R., Skerry T. M., Schaffler M. B. (1998) Intracortical remodeling in adult rat long bones after fatigue loading. Bone 23:275–281, doi:10.1016/S8756-3282(98)00104-5
Bloomfield S. A. (2001) Cellular and molecular mechanisms for the bone response to mechanical loading. Int. J. Sport Nutr. Exerc. Metab. 11(Suppl):S128–S136
Bonewald L. F. (2007) Osteocytes as dynamic multifunctional cells. Ann. N. Y. Acad. Sci. 1116:281–290, doi:10.1196/annals.1402.018
Burger E. H., Klein-Nulend J. (1999) Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J. 13(Suppl):S101–S112
Burger E. H., Klein-Nulend J., van der Plas A., Nijweide P. J. (1995) Function of osteocytes in bone—their role in mechanotransduction. J. Nutr. 125:2020S–2023S
Cane V., Marotti G., Volpi G., Zaffe D., Palazzini S., Remaggi F., Muglia M. A. (1982) Size and density of osteocyte lacunae in different regions of long bones. Calcif. Tissue Int. 34:558–563, doi:10.1007/BF02411304
Cooper R. R., Milgram J. W., Robinson R. A. (1966) Morphology of the osteon. An electron microscopic study. J. Bone Joint Surg. Am. 48:1239–1271
Cowin S. C. (1999) Bone poroelasticity. J. Biomech. 32:217–238, doi:10.1016/S0021-9290(98)00161-4
Cowin S. C., Weinbaum S., Zeng Y. (1995) A case for bone canaliculi as the anatomical site of strain generated potentials. J. Biomech. 28:1281–1297, doi:10.1016/0021-9290(95)00058-P
Dillaman R. M., Roer R. D., Gay D. M. (1991) Fluid movement in bone: theoretical and empirical. J. Biomech. 24(Suppl 1):163–177, doi:10.1016/0021-9290(91)90386-2
Dodd J. S., Raleigh J. A., Gross T. S. (1999) Osteocyte hypoxia: a novel mechanotransduction pathway. Am. J. Physiol. 277:C598–C602
Duriez R., Duriez J. (1981) Periosteocyte demineralization in disuse osteoporsis. The effect of calcitonin. Int. Orthop. 5:299–304, doi:10.1007/BF00271086
Eckmann D. M. (1998) Bolus contaminant dispersion in oscillating flow in curved tubes. J. Biomech. Eng. 120:238–244, doi:10.1115/1.2798307
Feng J. Q., Ward L. M., Liu S., Lu Y., Xie Y., Yuan B., Yu X., Rauch F., Davis S. I., Zhang S., Rios H., Drezner M. K., Quarles L. D., Bonewald L. F., White K. E. (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38:1310–1315, doi:10.1038/ng1905
Ferretti M., Muglia M. A., Remaggi F., Cane V., Palumbo C. (1999) Histomorphometric study on the osteocyte lacuno-canalicular network in animals of different species. II. Parallel-fibered and lamellar bones. Ital. J. Anat. Embryol. 104:121–131
Fornells P., Garcia-Aznar J. M., Doblare M. (2007) A finite element dual porosity approach to model deformation-induced fluid flow in cortical bone. Ann. Biomed. Eng. 35:1687–1698, doi:10.1007/s10439-007-9351-5
Fritton S. P., McLeod K. J., Rubin C. T. (2000) Quantifying the strain history of bone: spatial uniformity and self-similarity of low-magnitude strains. J. Biomech. 33:317–325, doi:10.1016/S0021-9290(99)00210-9
Fritton J. C., Myers E. R., Wright T. M., van der Meulen M. C. (2005) Loading induces site-specific increases in mineral content assessed by microcomputed tomography of the mouse tibia. Bone 36:1030–1038, doi:10.1016/j.bone.2005.02.013
Gaver D. P. 3rd, Solway J., Punjabi N., Elad D., Grotberg J. B., Gavriely N. (1992) Gas dispersion in volume-cycled tube flow. II. Tracer bolus experiments. J. Appl. Physiol. 72:321–331
Genetos D. C., Kephart C. J., Zhang Y., Yellowley C. E., Donahue H. J. (2007) Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell Physiol. 212:207–214, doi:10.1002/jcp.21021
Glasgow I., Aubry N. (2003) Enhancement of microfluidic mixing using time pulsing. Lab. Chip 3:114–120, doi:10.1039/b302569a
Glasgow I., Lieber S., Aubry N. (2004) Parameters influencing pulsed flow mixing in microchannels. Anal. Chem. 76:4825–4832, doi:10.1021/ac049813m
Gluhak-Heinrich J., Ye L., Bonewald L. F., Feng J. Q., MacDougall M., Harris S. E., Pavlin D. (2003) Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J. Bone Miner. Res. 18:807–817, doi:10.1359/jbmr.2003.18.5.807
Goffe W. L., Ferrier G. D., Rogers J. (1994) Global optimization of statistical functions with simulated annealing. J. Econom. 60:65–99, doi:10.1016/0304-4076(94)90038-8
Gross T. S., Akeno N., Clemens T. L., Komarova S., Srinivasan S., Weimer D. A., Mayorov S. (2001) Selected contribution: osteocytes upregulate HIF-1alpha in response to acute disuse and oxygen deprivation. J. Appl. Physiol. 90:2514–2519
Gross T. S., King K. A., Rabaia N. A., Pathare P., Srinivasan S. (2005) Upregulation of osteopontin by osteocytes deprived of mechanical loading or oxygen. J. Bone Miner. Res. 20:250–256, doi:10.1359/JBMR.041004
Gururaja S., Kim H. J., Swan C. C., Brand R. A., Lakes R. S. (2005) Modeling deformation-induced fluid flow in cortical bone’s canalicular-lacunar system. Ann. Biomed. Eng. 33:7–25, doi:10.1007/s10439-005-8959-6
Keanini R. G., Roer R. D., Dillaman R. M. (1995) A theoretical model of circulatory interstitial fluid flow and species transport within porous cortical bone. J. Biomech. 28:901–914, doi:10.1016/0021-9290(94)00157-Y
Klein-Nulend J., Semeins C. M., Ajubi N. E., Nijweide P. J., Burger E. H. (1995) Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts—correlation with prostaglandin upregulation. Biochem. Biophys. Res. Commun. 217:640–648, doi:10.1006/bbrc.1995.2822
Knothe Tate M. L., Knothe U. (2000) An ex vivo model to study transport processes and fluid flow in loaded bone. J. Biomech. 33:247–254, doi:10.1016/S0021-9290(99)00143-8
Knothe Tate M. L., Niederer P., Knothe U. (1998) In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117, doi:10.1016/S8756-3282(97)00234-2
Kufahl R. H., Saha S. (1990) A theoretical model for stress-generated fluid flow in the canaliculi-lacunae network in bone tissue. J. Biomech. 23:171–180, doi:10.1016/0021-9290(90)90350-C
Leddy H. A., Guilak F. (2003) Site-specific molecular diffusion in articular cartilage measured using fluorescence recovery after photobleaching. Ann. Biomed. Eng. 31:753–760, doi:10.1114/1.1581879
Lemaire T., Naili S., Remond A. (2006) Multiscale analysis of the coupled effects governing the movement of interstitial fluid in cortical bone. Biomech. Model. Mechanobiol. 5:39–52, doi:10.1007/s10237-005-0009-7
Li C. Y., Schaffler M. B., Wolde-Semait H. T., Hernandez C. J., Jepsen K. J. (2005) Genetic background influences cortical bone response to ovariectomy. J. Bone Miner. Res. 20:2150–2158, doi:10.1359/JBMR.050819
Li W., Zhou X., Novotny J. E., Wang L. (2007) Solute transport among osteocytes in live animals. J. Musculoskelet. Neuronal Interact. 7:366–367
Mak A. F., Zhang J. D. (2001) Numerical simulation of streaming potentials due to deformation-induced hierarchical flows in cortical bone. J. Biomech. Eng. 123:66–70, doi:10.1115/1.1336796
Marotti G., Ferretti M., Remaggi F., Palumbo C. (1995) Quantitative evaluation on osteocyte canalicular density in human secondary osteons. Bone 16:125–128, doi:10.1016/S8756-3282(94)00019-0
Marotti G., Remaggi F., Zaffe D. (1985) Quantitative investigation on osteocyte canaliculi in human compact and spongy bone. Bone 6:335–337, doi:10.1016/8756-3282(85)90325-4
Mi L. Y., Fritton S. P., Basu M., Cowin S. C. (2005) Analysis of avian bone response to mechanical loading—part one: distribution of bone fluid shear stress induced by bending and axial loading. Biomech. Model. Mechanobiol. 4:118–131, doi:10.1007/s10237-004-0065-4
Noble B. (2005) Microdamage and apoptosis. Eur. J. Morphol. 42:91–98, doi:10.1080/09243860500096248
Petrov N., Pollack S. R. (2003) Comparative analysis of diffusive and stress induced nutrient transport efficiency in the lacunar-canalicular system of osteons. Biorheology 40:347–353
Piekarski K., Munro M. (1977) Transport mechanism operating between blood supply and osteocytes in long bones. Nature 269:80–82, doi:10.1038/269080a0
Poole K. E., van Bezooijen R. L., Loveridge N., Hamersma H., Papapoulos S. E., Lowik C. W., Reeve J. (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 19:1842–1844
Qin Y. X., Lin W., Rubin C. (2002) The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann. Biomed. Eng. 30:693–702, doi:10.1114/1.1483863
Reits E. A., Neefjes J. J. (2001) From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 3:E145–E147, doi:10.1038/35078615
Remaggi F., Cane V., Palumbo C., Ferretti M. (1998) Histomorphometric study on the osteocyte lacuno-canalicular network in animals of different species. I. Woven-fibered and parallel-fibered bones. Ital. J. Anat. Embryol. 103:145–155
Rios H. F., Ye L., Dusevich V., Eick D., Bonewald L. F., Feng J. Q. (2005) DMP1 is essential for osteocyte formation and function. J. Musculoskelet. Neuronal Interact. 5:325–327
Robling, A. G., P. J. Niziolek, L. A. Baldridge, K. W. Condon, M. J. Allen, I. Alam, S. M. Mantila, J. Gluhak-Heinrich, T. M. Bellido, S. E. Harris, C. H. Turner. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 2007, 283:5866–5875
Schmidt S. M., M. J. McCready, and A. E. Ostafin. Effect of oscillating fluid shear on solute transport in cortical bone. J. Biomech. 38:2337–2343, 2005
Silvestrini, G., P. Ballanti, M. Sebastiani, M. Leopizzi, M. Di Vito, E. Bonucci. OPG and RANKL mRNA and protein expressions in the primary and secondary metaphyseal trabecular bone of PTH-treated rats are independent of that of SOST. J. Mol. Histol. 2007, 39:237–242
Smit T. H., Huyghe J. M., Cowin S. C. (2002) Estimation of the poroelastic parameters of cortical bone. J. Biomech. 35:829–835, doi:10.1016/S0021-9290(02)00021-0
Steck R., Niederer P., Knothe Tate M. L. (2003) A finite element analysis for the prediction of load-induced fluid flow and mechanochemical transduction in bone. J. Theor. Biol. 220w:249–259, doi:10.1006/jtbi.2003.3163
Su M., Jiang H., Zhang P., Liu Y., Wang E., Hsu A., Yokota H. (2006) Knee-loading modality drives molecular transport in mouse femur. Ann. Biomed. Eng. 34:1600–1606, doi:10.1007/s10439-006-9171-z
Takai E., Mauck R. L., Hung C. T., Guo X. E. (2004) Osteocyte viability and regulation of osteoblast function in a 3D trabecular bone explant under dynamic hydrostatic pressure. J. Bone Miner. Res. 19:1403–1410, doi:10.1359/JBMR.040516
Tatsumi S., Ishii K., Amizuka N., Li M., Kobayashi T., Kohno K., Ito M., Takeshita S., Ikeda K. (2007) Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5:464–475, doi:10.1016/j.cmet.2007.05.001
Verborgt O., Gibson G. J., Schaffler M. B. (2000) Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J. Bone Miner. Res. 15:60–67, doi:10.1359/jbmr.2000.15.1.60
Wang L., Ciani C., Doty S. B., Fritton S. P. (2004) Delineating bone’s interstitial fluid pathway in vivo. Bone 34:499–509, doi:10.1016/j.bone.2003.11.022
Wang L., Cowin S. C., Weinbaum S., Fritton S. P. (2000) Modeling tracer transport in an osteon under cyclic loading. Ann. Biomed. Eng. 28:1200–1209, doi:10.1114/1.1317531
Wang L., Fritton S. P., Cowin S. C., Weinbaum S. (1999) Fluid pressure relaxation depends upon osteonal microstructure: modeling an oscillatory bending experiment. J. Biomech. 32:663–672, doi:10.1016/S0021-9290(99)00059-7
Wang L., Fritton S. P., Weinbaum S., Cowin S. C. (2003) On bone adaptation due to venous stasis. J. Biomech. 36:1439–1451, doi:10.1016/S0021-9290(03)00241-0
Wang Y., Hutter K. (2001) Comparisons of numerical methods with respect to convectively dominated problems. Int. J. Numer. Meth. Fluids 37:721–745, doi:10.1002/fld.197
Wang L., Wang Y., Han Y., Henderson S. C., Majeska R. J., Weinbaum S., Schaffler M. B. (2005) In situ measurement of solute transport in the bone lacunar-canalicular system. Proc. Natl. Acad. Sci. USA 102:11911–11916, doi:10.1073/pnas.0505193102
Weinbaum S., Cowin S. C., Zeng Y. (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 27:339–360, doi:10.1016/0021-9290(94)90010-8
Westbroek I., Ajubi N. E., Alblas M. J., Semeins C. M., Klein-Nulend J., Burger E. H., Nijweide P. J. (2000) Differential stimulation of prostaglandin G/H synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem. Biophys. Res. Commun. 268:414–419, doi:10.1006/bbrc.2000.2154
You L., Cowin S. C., Schaffler M. B., Weinbaum S. (2001) A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech. 34:1375–1386, doi:10.1016/S0021-9290(01)00107-5
You L., Temiyasathit S., Lee P., Kim C. H., Tummala P., Yao W., Kingery W., Malone A. M., Kwon R. Y., Jacobs C. R. (2008) Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 42:172–179, doi:10.1016/j.bone.2007.09.047
You L. D., Weinbaum S., Cowin S. C., Schaffler M. B. (2004) Ultrastructure of the osteocyte process and its pericellular matrix. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 278:505–513, doi:10.1002/ar.a.20050
Zeng Y., Cowin S. C., Weinbaum S. (1994) A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann. Biomed. Eng. 22:280–92, doi:10.1007/BF02368235
Zhang X., Adamson R. H., Curry F. R., Weinbaum S. (2006) A 1-D model to explore the effects of tissue loading and tissue concentration gradients in the revised Starling principle. Am. J. Physiol. Heart Circ. Physiol. 291:H2950–H2964, doi:10.1152/ajpheart.01160.2005
Acknowledgment
This study was supported by grants from NIH/NIAMS (AR054385 and P20RR016458).
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix A: Estimation of the LCS Permeability and the Characteristic Fluid Relaxation Time
The hydraulic permeability of a single canaliculus k p has been estimated using a model of rectangular lattice of transverse fibers in the fluid field.65
where a 0 is the radius of the pericellular fibers (assumed to that of proteoglycan GAG side chain, 0.6 nm), and \({\Updelta}\) is the effective spacing of the fibers of the pericellular matrix (7 nm).
The tissue level permeability k is estimated from the anatomical features of the LCS, assuming a regular array of osteocytes and homogenous canalicular distribution as in Weinbaum et al.65:
where a is the radius of the osteocyte process, q is the ratio of the radius of the canaliculus (b) to the radius of the osteocytic process (a), d is the average spacing between two lacunae, N is the total number of canaliculi emanating from one lacuna, γ is a dimensionless length ratio between the canalicular radius and its associated boundary layer thickness, which is approximated to be the square root of the permeability of a single canaliculus \(\left(\gamma =b/{\sqrt{k_{\rm p}}}\right).\) A 1 and B 1 are defined as
where I 0, K 0, I 1 and K 1 are modified Bessel functions of the first and second kind.
The characteristic fluid relaxation time is defined as:
where μ is the viscosity of bone fluid and assumed to be that of sea water (μ = 1.06 × 10−3 kg/ms); r 0 is the radius of the periosteum. The value of τ r is found to be 6.76 s for the mid-shaft tibia of B6 mouse (r 0 = 0.57 mm) when the reported values of the LCS ultra- and micro-structures are used (i.e., a = 52 nm; b = 130 nm; N = 70; d = 30 μm).64,69 The sensitivity of τ r on fiber spacing, size of cell process and canalicular wall was performed previously, and τ r was found to be more sensitive to the fiber spacing.67
Appendix B: Fluid Pore Pressure, Pressure Gradient, and Canalicular Fluid Velocity
The intermittent dynamic force f(t) (see Fig. 1 and Eq. 1 in the text) can be expanded into a Fourier series:
where F is the peak-to-peak force and 2t 1 is the resting time and 2t 2 is the loading period and the coefficient for each term is defined as:
Using a set of parameters [R = r/r o; τ = t/τ r ; T = ωτ r ; ω = π/(t 1 + t 2); and P = 3 pA/(BTF)] and substituting the Fourier series of loading f(t), the poroelastic equation of the fluid pore pressure (Eq. 3 in the text) is rendered dimensionless:
where R, τ, T, and P are dimensionless radial position, time, frequency, and pressure respectively; ω is the principle angular loading frequency; F is the peak force of the dynamic loading; A is the cross-sectional area of the tibial mid-shaft, B is the Skempton parameter indicating the relative compressibility between the fluid and solid phases in bone (B = 0.53)9,10; r 0 is the exterior radius of the tibial mid-shaft, L is the offset of the loading force to the center of the bone, I is the moment of inertia of the bone cross-section along the medial-lateral axis, and A n are the coefficients in the Fourier expansion (Eq. B2).
The boundary conditions are zero pressure at the inner endosteal surface and a leaky outer periosteal surface (Eq. B4), as in previous models60,65,70:
where η is the coefficient with η = 0 corresponding to no leakage condition and η→∞ corresponding to free flow condition.
The analytical solution of dimensionless pressure is derived using complex function and separate variables as follows:
where
The gradients of the dimensionless pressure in the radial and circumferential directions are obtained from Eq. (B5):
The corresponding canalicular fluid velocities in the radial and circumferential directions are derived using Darcy’s law:
where p is the fluid pressure, P is the dimensionless pressure, and k p is the permeability of a single canaliculus (defined in Eq. A1 in Appendix A).
Appendix C: Unified Transport Equation for the Bone LCS
For the three-compartment transport model (shown in Fig. 3 in the text), oscillating fluid and solute flows occur in the idealized one-dimensional LCS pathway with varying cross-sectional area. A unified, compact form of the transport equation for all segments is derived here. A liner x coordinate is defined and originated from the central lacuna (Fig. 3).
In canaliculi (d e + d s ≤ |x | ≤ d + d e + d s)
The solute transport satisfies the modified diffusion-convection equation through a porous media
where C is the tracer concentration, u is the bulk flow velocity (−u θ in this case), and D is the diffusion coefficient of the tracer. The boundary conditions are
where C 0 is the tracer concentration that remains constant in the two source reservoirs. Initially, a linear distribution is assumed in the canaliculi connecting the reservoir and the photobleached lacuna, assuming a relative slow photobleaching process.64
where C b is the concentration inside the photobleached lacuna immediately after photobleaching.
Tapered Entrance (d s ≤ |x | ≤ d e + d s)
The connection between the canalicular channels and the photobleached lacuna is modeled as a tapered tube with expanding cross-sectional area. The cross-sectional area is a function of position x as:
where A c is the summation of the cross-sectional area of the connecting canaliculi. The expansion factor β describes the cross-section radius increment on unit axial distance. Since the tapered entrance connects with the lacuna at the other end, (A = A f, extracellular fluid space in the lacuna, when |x| = d s), β can be determined by
The flow velocity in this section decreases with the increasing cross-sectional area:
The solute conservation equation is
After substituting Eqs. (C6) and (C4) into Eq. (C7), we derive the following equation for tapered section:
The initial condition for this section is that it is photobleached as the overall lacuna, i.e.,
Photobleached Lacuna (|x | ≤ d s)
Assuming the cross-sectional area of the extracellular fluid space in the lacuna (A f) remains constant along its long axis, A f can be obtained from Eq. (C5) as
where \(\lambda _2 =\frac{d_{\rm e}}{d}.\) Therefore, the fluid velocity in this region will decrease and the convection-diffusion equation becomes
The initial condition is that the entire of the lacuna will be uniformly photobleached as follows:
Unified Dimensionless Equation
The above equations can be rendered dimensionless using the following dimensionless parameters (Eq. C13).
To obtain a compact form of the transport equations, we define two functions g and h that vary with spatial locations and reflect the influences of the cross-sectional area variation on convective velocity and mass flow rate:
The compact form of the transport equation is given as:
The initial and boundary conditions are
Rights and permissions
About this article
Cite this article
Zhou, X., Novotny, J.E. & Wang, L. Modeling Fluorescence Recovery After Photobleaching in Loaded Bone: Potential Applications in Measuring Fluid and Solute Transport in the Osteocytic Lacunar-Canalicular System. Ann Biomed Eng 36, 1961–1977 (2008). https://doi.org/10.1007/s10439-008-9566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-008-9566-0