Abstract
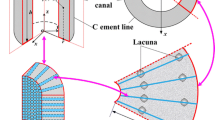
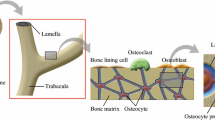
A theoretical model is developed to predict the fluid shear stress and streaming potential at the surface of osteocytic processes in the lacunar-canalicular porosity of an osteon when the osteon is subject to mechanical loads that are parallel or perpendicular to its axis. The theory developed in Weinbaumet al. (31) for the flow through a proteoglycan matrix in a canaliculus is employed in a poroelastic model for the osteon. Our formulation is a generalization of that of Petrovet al. (17). Our model predicts that, in order to satisfy the measured frequency dependence of the phase and magnitude of the SGP in macroscopic bone samples, the fiber spacing in the fluid annulus must lie in the narrow range 6–7 nm typical of the spacing of GAG sidechains along a protein monomer. The model predictions for the local SGP profiles in the osteon agree with the experimental observations of Starkebaumet al. (24). The theory predicts that the pore pressure relaxation time, τd, for a 150–300 μm diameter osteon with the foregoing matrix structure is approximately 0.03–0.13 sec, and that the amplitude of the mean fluid shear stress on the membrane of the osteocytic process at the mean areal radius of the osteon has a maximum at 28 Hz if τd = 0.06 sec. This maximum, which is independent of the magnitude of the loading, could be importantin vivo since the recent experiments of Turneret al. (28) and McLeodet al. (15) have a peak in the strain frequency spectrum between 20 and 30 Hz that also appears to be independent of the type (magnitude) of loading. Numerical predictions for the amplitude of the average fluid shear stress on the osteocytic membrane at the mean areal radius of the osteon show that the fluid shear stress associated with the low amplitude 20–30 Hz spectral strain component is at least as large as the average fluid shear stress associated with the high amplitude 1 Hz stride component, although the latter loading is an order of magnitude larger, and has a magnitude that lies within the middle of the range, 6–30 dynes/cm2, where fluid shear stresses in tissue culture studies with osteoblast monolayers have elicited an intracellular Ca++ response (31). The implications of these results for intracellular electrical communication are discussed.
Similar content being viewed by others
References
Adamson, R.H.; Clough, G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J. Physiol. 445:473–486; 1992.
Bianco, P.; Fisher, L.W.; Young, M.F.; Termine, J.D.; Robey, P.G. Expression and localization of the two small proteoglycans, biglycan and decorin, in human developing skeletal and non-skeletal tissues. J. Histochem. Histocytol. 38:1549–1563; 1990.
Buckwalter, J.A.; Rosenberg, L.C. Electron microscopic studies of cartilage proteoglycans—Direct evidence for the variable length of the chondroitin sulfate-rich region of proteoglycan subunit core protein. J. Biol. Chem. 257:9830–9839; 1982.
Buschmann, M.D.; Basser, P.J.; Grodzinsky, A.J. A microstructural model for swelling pressure and compressive modulus of tissue containing charged GAG chains: Comparison to Donnan theory, Bioengineering Conference. Langrana, N.A.; Friedman, M.H.; Grood, E.S., eds. ASME; 1993: pp. 80–83.
Cowin, S.C.; Weinbaum, S.; Zeng, Y. A case for bone canaliculi as the anatomical site of strain generated potentials. J. Biomechanics, in press.
Doty, S.B.; Schofield, B.H. Metabolic and structural changes within osteocytes of rat bone in: Talmage, B.V.; Munson, P.L., eds. Calcium, Parathyroid Hormone and the Calcitonins. Amsterdam: Excerpta Medica; 1972: pp. 353–364.
Frank, J.S.; Fogelman, A.M. Ultrastructure of the intima in WHHL and cholesterol-fed rabbit aortas prepared by ultrarapid freezing and freeze-etching. J. Lipid Res. 30:967–977; 1989.
Jeansonne, B.G.; Eagin, F.F.; McMinn, R.W.; Sheemaker, R.L.; Rehm, W.S. Cell to cell communication of osteoblasts. J. Dental Res. 58:1415–1423; 1979.
Johnson, M.W.; Chakkalakal, D.A.; Harper, R.A.; Katz, J.L.; Rouhana, S.W. Fluid flow in bone. J. Biomechanics 11:881–885; 1982.
Kamiya, A.; Togawa, T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 239:H14-H21; 1980.
Kamiya, A.; Bukhari, R.; Togawa, T. Adaptive regulation of wall shear stress optimizing vascular tree function. Bull. Math. Biol. 46:127–137; 1984.
Kufahl, R.H.; Saha, S. A theoretical model for stressgenerated fluid flow in the canaliculi-lacunae network in bone tissue. J. Biomechanics 23:171–180; 1990.
Langille, B.L.; O'Donnell, F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231:405–407; 1986.
Lowenstein, W.R. The cell-to-cell channel of gap junctions. Cell 48:725–726; 1987.
McLeod, K.J.; Rubin, C.T. Strain oscillations in functionally loaded bone: A species independent determinant of skeletal morphologo. J. Biomechanics, in press.
Michel, C. Capillary permeability and how it may change. J. Physiol. 404:1–29; 1988.
Petrov, N., Pollack, S., and Blagoeva R. A discrete model for streaming potentials in a single osteon. J. Biomechanics 22:512–521; 1989.
Piekarski, K.; Munro, M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature 269:80–82; 1977.
Prince, C.W.; Rahemtulla, F.; Butler, W.T. Incorporation of [35S]-sulphate into glycosamino-glycans by mineralized tissuesin vivo. Biochem. J. 224:941–945; 1984.
Reich, K.M.; Frangos, J.A. Effect of flow on prostaglandin E2 and inositol triphosphate levels in osteoblasts. Am. J. Physiol. 261:C428-C432; 1991.
Robey, P.G.; Bianco, P.; Termine, J.D. The cellular biology and molecular biochemistry of bone formation.In Disorders of Bone and Mineral Metabolism, edted by F.L. Coe and M.J. Favus, New York: Raven Press, 1992, pp. 241–263.
Salzstein, R.A.; Pollack, S.R.; Mark, A.F.T.; Petrov, N. Electromechanical potentials in cortical bone-I. A continuum approach. J. Biomechanics 20:261–270; 1987.
Salzstein, R.A.; Pollack, S.R. Electromechanical potentials in cortical bone-II. Experimental analysis. J. Biomechanics 20:271–280; 1987.
Starkebaum, W.; Pollack, S.R.; Korostoff, E. Microelectrode studies of stress-generated potentials in four-point bending of bone. J. Biomed. Mat. Res. 13:729–751; 1979.
Scott, G.C.; Korostoff, E. Oscillatory and step response electromechanical phenomena in human and bovine bone. J. Biomechanics 23:127–143; 1990.
Tanaka, T.; Sakano, A. Differences in permeability of microperoxidase and horseradish peroxidase into alveolar bone of developing rats. J. Dental Res. 64:870–876; 1985.
Timoshenko, S.P.; Goodier, J.N. Theory of elasticity. New York. McGraw Hill, 1970, 567 pp.
Tsay, R. Y.; Weinbaum, S. Viscous flow in a channel with periodic cross-bridging fibers: Exact solutions and Brinkman approximation. J. Fluid Mech. 226:125–148; 1991.
Turner, C.H.; Yoshikawa, T.; Forwood, M.R.; Sun, T.C.; Burr, D.B. Functional significance of high frequency components of bone strain in dogs. Trans. ORS 126:636–652; 1993.
Wassermann, F.; Yaeger, J.A. Fine structure of the osteocyte capsule and of the wall of the lacunae in bone. Zeitschrift für Zellforschung 67:636–652; 1965.
Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading induced bone fluid shear stresses. J. Biomechanics 273:339–360, 1994.
Williams, J.L.; Iannotti, J.P.; Ham, A.; Bleuit, J.; Chen, J.H. Effects of fluid shear stress in bone cells. J. Biorheology 31:163–170, 1994.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zeng, Y., Cowin, S.C. & Weinbaum, S. A fiber matrix model for fluid flow and streaming potentials in the canaliculi of an osteon. Ann Biomed Eng 22, 280–292 (1994). https://doi.org/10.1007/BF02368235
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02368235