Abstract
Brood survival as an expression of breeding success is a component of bird fitness and the principal parameter underlying bird population dynamics. The main factor limiting brood survival is predation pressure, and birds have developed anti-predator strategies by selecting safer places for building nests and breeding. Here, we examined whether nest location affected the level of predation pressure. To do so, we analyzed brood survival in a Red-backed Shrike (RBS) Lanius collurio population breeding in the low-intensive agricultural landscape of eastern Poland. The mean survival probability for the whole nesting period was 0.540 and was higher for the incubation period (0.787) than for the nestling period (0.696). Cox’s proportional hazard model did not show any significant traits describing the location of the nesting shrub or the nest’s position in the shrub that could have affected RBS brood survival. We consider the composition of the local predator community to influence brood survival more than the location of a nest in the bush. The Eurasian Magpie Pica pica is mainly responsible for RBS brood predation. Red-backed Shrikes do not defend their broods against Magpies using acoustic signals and direct contact. Since Magpies search the bushes thoroughly, the shrikes’ nest concealment strategies are ineffective in the face of this predator.
Zusammenfassung
Das Überleben der Brut beim Neuntöter Lanius collurio in Ostpolen
Das Überleben der Brut als Ausdruck des Bruterfolgs ist eine wichtige Komponente der Fitness von Vögeln und der wichtigste Parameter bei der Dynamik von Vogelpopulationen. Der wichtigste, das Überleben der Brut einschränkende Faktor ist dabei der von Räubern erzeugte Druck, und Vögel haben Strategien zum Schutz vor Räubern entwickelt, indem sie möglichst sichere Stellen für den Nestbau und die Brut auswählen. Wir untersuchten, ob es einen Einfluss des Neststandorts auf das Ausmaß des Drucks durch Räuber gab. Hierfür haben wir das Überleben der Brut in einer Population des Neuntöters (Lanius collurio) ermittelt, die in einer nur schwach landwirtschaftlich genutzten Gegend in Ostpolen brütet. Die mittlere Überlebenswahrscheinlichkeit über die gesamte Brutzeit hinweg betrug 0,540 und war für die Brutzeit (0,787) höher als für die Nestlingszeit (0,696). Das Cox'sche Proportional-Gefährdungs-Modell erbrachte keine signifikanten Merkmale, die hätten beschreiben können, wie der Standort der Büsche oder die Position eines Nests in den Büschen das Überleben der Neuntöter-Brut beeinflusst haben könnten. In erster Linie ist die Elster (Pica pica) für den Brutraub bei Neuntötern verantwortlich, und Neuntöter verteidigen ihre Brut vor den Elstern nicht durch Lautäußerungen oder einen direkten Kontakt. Da die Elstern das Gebüsch stets gründlich durchsuchen, sind die Strategien der Neuntöter zum Verstecken der Nester bei diesem Räuber wirkungslos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nest survival is a component of bird fitness and the main parameter of bird population dynamics (Newton 1998; Cresswell 2010). Predation accounts for as much as 70% of nest failures, especially in open-cup nesting species (Martin 1993; Colombelli-Négrel and Kleindorfer 2009; Ibáñez-Álamo et al. 2015). Birds have evolved behavioral strategies to avoid predation, e.g., the selection of nest sites and the active defence of nests (Weidinger 2002; Kleindorfer 2007), and shrikes are also capable of physically transferring their nestlings (Yosef and Pinshow 1988). The success of a bird’s nest is influenced by its micro- and macro-characteristics (Hansell 2000), and the most frequently examined nest-site characteristics, especially in tree-nesting species, are nest height, the degree of nest concealment and the density of the surrounding vegetation (Weidinger 2002; Batary et al. 2014). A well-hidden nest may hamper a predator’s search efforts and attenuate the auditory, olfactory or visual cues emitted by potential prey (Martin and Roper 1988; Martin 1993). However, while some authors consider that the relationship between nest-site selection and nesting success is not always significant (Davis 2005; Jara et al. 2020), others doubt whether birds are capable of maximizing fitness through nest-site selection (Latif et al. 2012; Soanes et al. 2015).
True Shrikes (Laniidae spp.) are birds whose broods are subject to strong predation pressure, mainly on the part of corvids (Lefranc 2022), and usually only ca. 50% of broods are successful (e.g., Esely and Bollinger 2001; Antczak et al. 2004; Nikolov 2005; Golawski and Meissner 2008; Campos et al. 2011). It is generally believed that in the case of shrikes, siting the nest appropriately is one of the most important ways of minimizing the loss of reproductive investment as a result of predation (Müller et al. 2005; Matsui and Takagi 2012). Such dependence has been demonstrated in some species: nests located lower down and better hidden in foliage had a higher breeding success than poorly concealed nests in the same micro-environment (Takagi and Abe 1996; Endo 2012). Breeding success in shrikes has been found to depends on the distance to a forest edge (Matyjasiak 1995), and differences have also been found between nests located in linear and patchy clumps of shrubs (Wozna et al. 2017). It is often emphasized, moreover, that shrikes frequently build their nests in thorny bushes, thereby achieving a higher breeding success because the thorns restrict access to predators (Tryjanowski et al. 2000; Campos et al. 2011; Aghanajafizadeh et al. 2014). However, these characteristics of nest concealment have not always been associated with superior brood parameters (see Isenmann and Fradet 1998; Golawski and Mitrus 2008; Campos et al. 2011). What is important in this type of research is that reproductive success is usually expressed as a binary variable (nest with or without reproductive success) or as the number of fledglings, but such an approach does not take into account that the probability of breeding success in a population during the breeding season as a function of time.
Here, we present the results of research on a Red-backed Shrike Lanius collurio population based on long-term data and a large sample size, in which, by analyzing brood survival, we examined the relationship between the choice of nest site and reproductive success as mediated exclusively by predation. This method enables to estimate the survival function of broods during the breeding season. The research aims were: (1) to describe how predation pressure affects the reproductive success of the Red-backed Shrike during the incubation and nestling stages; (2) to establish the critical period of the reproductive cycle that influences reproductive success; and (3) to investigate whether the main factors linked to the nest site affect reproductive success. We expected that the better-hidden broods, i.e., located in clusters of bushes, deep within a bush and in thorny bushes, would survive better than broods that were more exposed and in thornless bushes, and thus readily accessible to potential predators.
Materials and methods
Study species
The Red-backed Shrike (RBS) is a small, territorial passerine bird species widely distributed in Europe and western Asia, with a decreasing population trend (BirdLife International 2023). It is a long-distance migrant, which arrives at its breeding grounds from Africa in April/May (Lefranc 2022). In eastern Poland, the majority of the population inhabits agricultural landscapes, breeding on the edge of woods, in clumps of trees, in orchards and currant plantations (Golawski and Meissner 2008). The breeding season usually starts in mid-May and extends into August. Three–seven eggs are laid and incubated for 14–15 days. The nestlings remain in the nest for the next ca. 15 days, and fledglings stay around the nest for another 2–4 weeks (Lefranc 2022). This species is normally single-brooded, but in the case of first-brood failure, replacement clutches are regularly laid (Antczak et al. 2009). The RBS is a mainly insectivorous species (Tryjanowski et al. 2003; Morelli et al. 2015).
Study area
The study was conducted in the agricultural landscape around the town of Siedlce in eastern Poland (52.14° N, 21.93° E). Arable fields predominated in this area (53.5%), mainly with crops of rye Secale sp. and maize Zea mays; meadows and pastures covered 21.1% and 2.2% was fallow land. Woodlands, apple Malus sp. orchards and currant Ribes sp. plantations were also present in addition to these open habitats. Single bushes and trees are scattered over the area, and there are also groves of various sizes. The species composition of both trees and shrubs is diverse. Deciduous species predominate. The structure of land use generally did not change significantly during the period of the study (UMWM 2020). The sole discernible change observed was an increase in the area of maize cultivation, which occurred at the expense of cereal crops. Both cereal crops and maize cultivation were not used as feeding areas for shrikes, so these changes were insignificant to the described population. In the study area, there are two corvids species—Eurasian Magpie Pica pica and European Jay Garrulus glandarius—which are generally recognized as predators that significantly affect RBS breeding populations (Roos and Part 2004; Müller et al. 2005). Neither species nests in the study area, but both fly in regularly from the surrounding area, especially the Magpie. Since the study area is generally an open area, and the Jay is mainly a forest and forest edge species, the Magpie is considered the most dangerous predator of this particular RBS population (Golawski and Golawska 2019).
Bird data
We conducted this study in the 1999–2003 and 2012–2021 breeding seasons. In each season, the fieldwork started in early May, when the first shrikes arrived and ended in July/August, when the last pairs departed with their young. We searched for RBS nests, checking possible favorable nesting locations and observing the birds’ behavior, e.g., a male feeding the incubating female. As we found most nests during the egg-laying or the early incubation phase, we calculated the clutch initiation date on the basis of the number of eggs laid (one egg is laid per day). If a clutch was being incubated, the advancement was based on the water test of the eggs (Wesołowski 1986). In this test, eggs just starting to incubate lie flat on the bottom of the water container; after a time, they start to stand on end and then float until they protrude above the water surface in the container. We found 83 (28%) nests at the nestling stage, where we weighed the nestlings and estimated their age according to the age–body mass relationship, described for this species based on data gathered in natural conditions (Diehl 1971). We assumed that the mean brood duration was 32 days, including the egg-laying and incubation periods (17 days) and the nestling stage (15 days). We checked these nests every 4–5 days to minimize the impact of the observer’s intrusion on nest survivorship (Golawski and Zduniak 2022). We expressed nesting success as nests with at least one fledgling. We analyzed only successful nests and those nests that were totally lost as a result of predation, i.e., when all the eggs failed or all the nestlings died or when remains of eggs or nestlings were found in or under the nest. Excluded from the analysis were data relating to broods lost because of bad weather or farming activities, or which had been abandoned by the parents as a result of a researcher being at the nest; these factors accounted for 13.9% of all brood losses (see Golawski and Zduniak 2022). Furthermore, the dataset contains only records relating to first broods, i.e., those initiated before 10 June (for an identical approach, see Tryjanowski 2002; Golawski and Golawska 2023). Our dataset thus consists of 296 broods out of the total of 442 recorded. The level of philopatry in the population studied was very low (Tryjanowski et al. 2007), so the probability that the same birds bred in this area in consecutive years was likewise very low. The average breeding density in this population was 2.08 (SE = 0.27; n = 15 years) pair/10 ha.
Nest-site characteristics
We used four variables, i.e., measures of brood concealment, to describe the nest sites, because the use of a single measure of nest concealment as an indication of predation risk is not deemed very credible (see Remeš 2005). The first was the position of the nest bush as a single one (at least 20 m away from its nearest neighbor) or located in a clump/row of trees/bushes (CLUSTER). The second trait was the type of shrub hosting the nest. This was subdivided into two groups: shrubs with thorns (conifers were included in this category) and without thorns (THORNS). Thorny plants included wild pear Pyrus pyraster, common buckthorn Rhamnus catharticus, hawthorn Crataegus sp., blackberry Rubus sp., Prunus species, dog rose Rosa canina, Norway spruce Picea abies and juniper Juniperus communis. The third variable was the distance between the nest and the edge of the host shrub measured with an accuracy of 1 cm (DISTANCE), which we took to be a measure of nest concealment by leaves. The fourth factor was the height of the nest above the ground (HEIGHT), determined with a measuring tape accurate to 1 cm up to the highest part of the nest.
Data analysis
Overall, the material analyzed included a cohort of 296 broods (mean per year = 19.7 ± 3.1 broods). We calculated brood survival based on the changes in the number of active nests observed in the study area during the breeding season and analyzed using life tables (Kleinbaum 1996). This method estimates survival functions where the life history of broods during a particular breeding season is known. The major advantage of using life tables is that this method assumes survival to be a function of time (Kleinbaum 1996; Langowska and Zduniak 2020), which is not the case when one analyses breeding success expressed as the simple proportion of surviving broods. This enables the critical time points during the study period to be identified, in this case, during the breeding season of the population studied (Zduniak 2010; Zduniak et al. 2011). We estimated the survival time of each brood from the first egg-laying date to the date of the last visit to each nest and calculated the day when eggs or nestlings failed as being halfway between two consecutive nest visits.
We applied Cox’s proportional hazards model (Cox 1972) to determine the possible effect of certain factors on brood survival. Although not often used by ecologists, this method is suitable for studying diverse ecological phenomena occurring in time and space (Muenchow 1986; Moya-Larano and Wise 2000; Reino et al. 2009). This model assumes that the hazard rate (a component of life tables, defined as the probability per time unit that an individual which has survived to the beginning of a specific interval will not survive to the end of that interval) is a function of the independent variables. With this model, one can estimate the regression coefficients for independent variables, including categorical factors. In this case, the variables with positive coefficients for a given category (e.g., nests in shrubs without thorns as the level of effect) are associated with decreased survival compared to the reference category (e.g., nests in thorny shrubs), whereas variables with negative coefficients for a particular category are associated with increased survival compared to the reference category. Furthermore, variables with positive coefficients are associated with a higher risk of failure and lower survival and variables with negative coefficients with a lower risk of death and enhanced survival.
With the aid of this model, we tested several variables describing nest locations that might influence brood survival as defined above: CLUSTER, THORNS, DISTANCE and HEIGHT. In the model, we also controlled the time of breeding (TIME), which is the time of brood initiation (i.e., the first egg-laying date) expressed as the number of days after 30 April and standardized to the median date for the particular breeding season. Owing to the considerable variation in the breeding success of the Red-backed Shrike (e.g., Hušek et al. 2010; Wozna et al. 2017), the model included sample stratification by the year of the study. We estimated the standard errors using 5000 bootstrap repetitions. To compare survival between the incubation and nestling-rearing periods, we applied Gehan’s generalized Wilcoxon test (Gehan 1965). We performed all calculations using IBM SPSS Statistics for Windows, version 28.0 (IBM Corp. Released 2020). Throughout the text, mean values are given with standard errors (± SE).
Results
We found 213 nests (72.0% of the 296) in a clump or row of bushes or trees. Furthermore, 219 nests (74.0%) were in bushes without thorns. The mean distance between a nest and the edge of the host shrub was 30.9 ± 2.1 cm (range 0–300 cm), and the mean height of a nest above the ground was 104.6 ± 5.0 cm (range 4–900 cm).
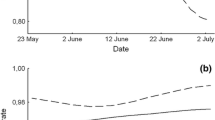
Overall, the mean nest survival rate for the whole nesting period (32 days, from egg laying to fledging) was 0.540 ± 0.029 (n = 296, Fig. 1), differing between the years (chi-square = 29.82, df = 15, p = 0.008) and varying between 0.286 ± 0.170 and 0.756 ± 0.067. Furthermore, the mean survival for the incubation period (until day 17; 0.787 ± 0.024; n = 296) was higher than for the nestling-rearing period (from day 18 to day 32; 0.696 ± 0,030; n = 230; Gehan–Wilcoxon test, test value = 3.42, p < 0.001). The magnitude of reproductive success (expressed as nests with at least one fledgling) was determined at both the incubation and nestling-rearing stages. However, the period with the highest hazard rate occurred around 8 days after the nestlings had hatched (Fig. 2). The predators were mainly corvids; only a few times did we find egg shells chewed by mammals.
Cox’s proportional hazard model with all covariates analyzed was significant (χ2 = 21.00, df = 5, p < 0.001), but only the time of brood initiation had a significant influence on nest survival (Table 1). The probability of nest failure increased with the advancement of the breeding season (Fig. 3).
Discussion
The results of our research showed that only one factor—brood initiation—affected the probability of nest survival, and the probability of nest failure increased as the breeding season advanced. It is generally considered that earlier breeding RBS pairs achieve a higher breeding success than later breeding ones (Müller et al. 2005; Wozna et al. 2017). Lower levels of breeding success as a result of later arrival at the breeding grounds have been demonstrated in many species. Individuals arriving earlier are usually older or more experienced (Verhulst and Nilsson 2008) and potentially have more opportunities to occupy better-quality habitats and territories (Weggler 2000; Kokko et al. 2006). In the study area, a significant part of the same sites (territories) was occupied every year. However, it is difficult to determine whether a given area was occupied year-on after-year by the same birds, as their rate of philopatry was low, probably because of the many suitable nesting sites in the surroundings (Tryjanowski et al. 2007).
The mean survival probability of the whole nesting period (0.540) varied greatly between seasons (0.286–0.756), which is typical for this species (Horvath et al. 2000; Hušek et al. 2010; Hemerik et al. 2015). These differences may be due to the number of predators in the study area (Roos and Part 2004; Polak 2014), as well as the weather conditions governing predator activity (Golawski and Golawska 2019). Such a large difference in achieved reproductive output between seasons shows that reliable estimates of population breeding parameters should be based on long-term data. The mean nest survival that we recorded in our study is consistent with long-term breeding success data on RBS obtained elsewhere (Tryjanowski 2002; Hušek et al. 2010; Schaub et al. 2011), as well as on other Lanius species (Tyler 1995; Antczak et al. 2004; Nikolov 2005; Campos et al. 2011).
Survival analysis showed that the hazard rate for predation during the egg-laying and incubation periods was lower than in the nestling-rearing stage. Despite the statistically significant results, the difference in the probability of survival between the periods compared (11.6%) was biologically not very important. Also, Polak (2016) stated that the daily survival rate of the RBS was relatively stable throughout the reproductive period. A similar level of brood survival at different stages was shown by Keynan and Yosef (2010) for Southern Grey Shrike Lanius meridionalis and by Yosef (2000) for Loggerhead Shrike Lanius ludovicianus. Some RBS vigorously defend broods, especially at the nestling stage, which results in better brood survival in this period (Tryjanowski and Goławski 2004; Polak 2014). This suggests that losses during the nestling-rearing period should be lower than during incubation. However, when parents are feeding the nestlings, they make frequent visits to the nest, thus revealing its location and making it easier for predators to find (Martin et al. 2000). This was probably reflected in the our results of research. We did not find that the four variables describing a nest’s position in a bush and the location of the bush influenced its survival probability. However, data from other authors indicate that the location of a RBS nest may affect its survival. In western Poland, for example, pairs nesting in clumps of shrubs achieved a significantly higher level of nesting success than those nesting in rows of bushes, probably because predators move and forage along linear landscape features; nests situated in these latter structures are thus more vulnerable to predation (Wozna et al. 2017). Many authors have found that breeding success is higher in nests better covered with leaves (the height of the nest above the ground is also related to this) than in more conspicuous ones (Jakober and Stauber 2002; Müller et al. 2005; Hollander et al. 2015; Polak 2016). On the other hand, shrikes are aggressive birds (Polak 2016) and they may locate their nests in such a way as to get relatively early warning of an approaching predator, so such a site need not necessarily be very well hidden. This makes for an earlier escape from the nest and, if need be, the birds can start to defend it before the predator reaches it. Individual birds may therefore benefit from keeping potential predators in view, and nest-site selection may in fact be a trade-off between concealment and visibility (Gotmark et al. 1995). It is possible, however, that the measure of nest concealment that we adopted, i.e., the distance of the nest from the edge of the bush, does not reflect its real concealment, and that the above-cited results based on more accurate measurements of nest visibility are more reliable. The location of shrike nests in thorny bushes, which restrict the access of predators, is frequently emphasized (Sooderstroom et al. 1998; Tryjanowski et al. 2000; Campos et al. 2011). Like Müller et al. (2005) and Wozna et al. (2017), we failed to demonstrate a relationship between nesting success and the thorniness of the nest site. Perhaps the lack of differences in breeding success in these two types of shrubs is due to the species composition of predators, because thorny bushes tend to favor mammalian predation (Tryjanowski et al. 2000); whereas, the predators in the area we studied were mainly corvids.
To summarize, our results show that none of the RBS nest-site choice strategies described above affected breeding success, as expressed by nest survival. In our opinion, whether the location of a nest affects brood survival during the breeding season depends on the composition of the local predator community. In the case of this RBS population, the most dangerous predator is the Eurasian Magpie because it cannot be effectively chased away from the nest (Roos and Part 2004; Veselý et al. 2022); in fact, a Magpie is rarely if ever attacked by shrikes (Syrová et al. 2016). As the Magpie makes a thorough search of bushes (Krüger et al. 2018), the shrikes’ nest concealment strategies are ineffective in the face of this predator. Our study has shown that the factors limiting predation of RBS nests, highlighted in many studies, do not necessarily apply in every part of this species' range. Our findings indicate that the shrikes' breeding success is mediated by specific local conditions.
Data availability
Not applicable.
References
Aghanajafizadeh S, Eslami K, Abbasian H, Heydari F (2014) Nest site selection by the Woodchat Shrike (Lanius senator) in Boroyeh Wildlife Sanctuary, Inner Iran. Zool Middle East 60:20–23
Antczak M, Hromada M, Grzybek J, Tryjanowski P (2004) Breeding biology of the Great Grey Shrike Lanius excubitor in W Poland. Acta Ornithol 39:9–14
Antczak M, Golawski A, Kuzniak S, Tryjanowski P (2009) Costly replacement—how do different stages of nest failure affect clutch replacement in the red-backed shrikes Lanius collurio? Ethol Ecol Evol 21:127–136
Batary P, Fronczek S, Normann C, Scherber C, Tscharntke T (2014) How do edge effect and tree species diversity change bird diversity and avian nest survival in Germany’s largest deciduous forest? For Ecol Manag 319:44–50
BirdLife International (2023) Species factsheet: Lanius collurio. Downloaded from http://www.birdlife.org on 14/01/2023
Campos F, Santamaria T, Gutierrez-Corchero F, Hernandez MA, Mas P (2011) Breeding success of Southern Grey Shrikes Lanius meridionalis in agricultural areas: the influence of nest site characteristics. Acta Ornithol 46:29–36
Colombelli-Négrel D, Kleindorfer S (2009) Nest height, nest concealment, and predator type predict nest predation in Superb Fairy-wrens (Malurus cyaneus). Ecol Res 24:921–928
Cox DR (1972) Regression models and life tables. J R Stat Soc 34:187–220
Cresswell W (2010) Predation in bird populations. J Ornithol 152:251–263
Davis SK (2005) Nest-site selection patterns and the influence of vegetation on nest survival of mixed-grass prairie passerines. Condor 107:605–616
Diehl B (1971) Productivity investigation of two types of meadows in the Vistula valley. Ekol Pol 19:235–248
Endo S (2012) Nest-site characteristics affect probability of nest predation of Bull-headed Shrikes. Wilson J Ornithol 124:513–517
Esely JD Jr, Bollinger EK (2001) Habitat selection and reproductive success of Loggerhead Shrikes in northwest Missouri: a hierarchical approach. Wilson Bull 113:290–296
Gehan EA (1965) A generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika 52:203–223
Golawski A, Golawska S (2019) Weather and predation pressure: The case of the Red-backed Shrike (Lanius collurio). Acta Zool Acad Sci Hung 65:371–379
Golawski A, Golawska S (2023) Delayed egg-laying in Red-backed Shrike Lanius collurio in relation to increased rainfall in east-central Poland. Int J Biometeorol 67:717–724
Golawski A, Meissner W (2008) The influence of territory characteristics and food supply on the breeding performance of the Red-backed Shrike (Lanius collurio) in an extensively farmed region of eastern Poland. Ecol Res 23:347–353
Golawski A, Mitrus C (2008) What is more important: nest-site concealment or aggressive behaviour? A case study of the red-backed shrike, Lanius collurio. Folia Zool 57:403–410
Golawski A, Zduniak P (2022) Influence of researcher experience and fieldwork intensity on the probability of brood losses in sensitive species: The case of the Red-backed Shrike Lanius collurio. J Nat Conserv 69:126249
Gotmark F, Blomqvist D, Johansson OC, Bergkvist J (1995) Nest site selection: a trade-off between concealment and view of the surroundings? J Avian Biol 26:305–312
Hansell MH (2000) Bird nests and construction behaviour. Cambridge University Press
Hemerik L, Geertsma M, Waasdorp S, Middelveld RP, van Kleef H, Klok TC (2015) Survival, reproduction, and immigration explain the dynamics of a local Red-backed Shrike population in the Netherlands. J Ornithol 156:35–46
Hollander FA, van Dyck H, San Martin G, Titeux N (2015) Nest predation deviates from nest predator abundance in an ecologically trapped bird. PLoS ONE 10:e0144098
Horvath R, Farkas R, Yosef R (2000) Nesting ecology of the Redbacked Shrike (Lanius collurio) in northeastern Hungary. Ring 22:127–132
Hušek J, Weidinger K, Adamík P, Hlavatý L, Holáň V, Sviečka J (2010) Analysing large-scale temporal variability in passerine nest survival using sparse data: a case study on Red-backed Shrike Lanius collurio. Acta Ornithol 45:43–49
Ibáñez-Álamo JD, Magrath RD, Oteyza JC, Chalfoun AD, Haff TM, Schmidt KA, Thomson RL, Martin TE (2015) Nest predation research: recent findings and future perspectives. J Ornithol 156:247–262
IBM Corp Released IBM (2020) SPSS Statistics for Windows, Version 28.0. IBM Corp, Armonk
Isenmann P, Fradet G (1998) Nest site, laying period, and breeding success of the Woodchat Shrike (Lanius senator) in Mediterranean France. J Ornithol 139:49–54
Jakober H, Stauber W (2002) Why do red-backed shrikes not build better concealed nests? J Ornithol 143:397–404
Jara RF, Crego RD, Samuel MD, Rozzi R, Jiménez JE (2020) Nest-site selection and breeding success of passerines in the world’s southernmost forests. PeerJ 8:e9892
Keynan O, Yosef R (2010) Annual precipitation affects reproduction of the Southern Grey Shrike (Lanius meridionalis). Wilson J Ornithol 122:334–339
Kleinbaum DG (1996) Survival analysis. Springer, New York
Kleindorfer S (2007) Nesting success in Darwin’s Small Tree Finch, Camarhynchus parvulus: evidence of female preference for older males and more concealed nests. Anim Behav 74:795–804
Kokko H, Gunnarsson TG, Morrell LJ, Gill JA (2006) Why do female migratory birds arrive later than males? J Anim Ecol 75:1293–1303
Krüger H, Väänänen VM, Holopainen S, Nummi P (2018) The new faces of nest predation in agricultural landscapes—a wildlife camera survey with artificial nests. Eur J Wildl Res 64:76
Langowska A, Zduniak P (2020) No direct contact needed for drones to shorten workers lifespan in honey bee. J Apic Res 59(1):88–94
Latif QS, Heath SK, Rotenberry JT (2012) How avian nest site selection responds to predation risk: testing an ‘adaptive peak hypothesis.’ J Anim Ecol 81:127–138
Lefranc N (2022) Shrikes of the World. Bloomsbury Publishing, London
Martin TE (1993) Nest predation and nest sites: new perspectives on old patterns. Bioscience 43:523–532
Martin TE, Roper JJ (1988) Nest predation and nest site selection in a western population of the hermit thrush. Condor 90:51–57
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc R Soc B Biol Sci 267:2287–2293
Matsui S, Takagi M (2012) Predation risk of eggs and nestlings relative to nest-site characteristics of the bull-headed shrike Lanius bucephalus. Ibis 154:621–625
Matyjasiak P (1995) Breeding ecology of the Red-backed Shrike in Poland. Proc West Found Vertebr Zool 6:228–234
Morelli F, Bussière R, Golawski A, Tryjanowski P, Yosef R (2015) Saving the best for last: Differential usage of impaled prey by red-backed shrike (Lanius collurio) during the breeding season. Behav Proc 119:6–13
Moya-Larano J, Wise DH (2000) Survival regression analysis: a powerful tool for evaluating fighting and assessment. Anim Behav 60:307–313
Muenchow G (1986) Ecological use of failure time analysis. Ecology 67:246–250
Müller M, Pasinelli G, Schiegg K, Spaar R, Jenni L (2005) Ecological and social effects on reproduction and local recruitment in the red-backed shrike. Oecologia 143:37–50
Newton I (1998) Population limitation in birds. Academic, London
Nikolov BP (2005) Reproductive success of the Woodchat Shrike (Lanius senator) in Western Bulgaria. Ornis Fenn 82:73–80
Polak M (2014) Protective nesting association between the Barred Warbler Sylvia nisoria and the Red-backed Shrike Lanius collurio: an experiment using artificial and natural nests. Ecol Res 29:949–957
Polak M (2016) Comparative breeding ecology, nest survival, and agonistic behaviour between the Barred Warbler and the Red-backed Shrike. J Ornithol 157:747–758
Reino L, Moya-Larano J, Antonio Heitor AC (2009) Using survival regression to study patterns of expansion of invasive species: will the common waxbill expand with global warming? Ecography 32:237–246
Remeš V (2005) Nest concealment and parental behaviour interact in affecting nest survival in the blackcap (Sylvia atricapilla): an experimental evaluation of the parental compensation hypothesis. Behav Ecol Sociobiol 58:326–332
Roos S, Part T (2004) Nest predators affect spatial dynamics of breeding red-backed shrikes (Lanius collurio). J Anim Ecol 73:117–127
Schaub M, Jakober H, Stauber W (2011) Demographic response to environmental variation in breeding, stopover and non-breeding areas in a migratory passerine. Oecologia 167:445–459
Soanes R, Peters A, Delhey K, Doody JS (2015) The influence of nest site choice and predator sensory cues on nest success in the Crimson Finch (Neochmia phaeton). Emu 115:317–325
Sooderstroom B, Part T, Erikson S, Karlsson P (1998) The effect of breeding habitat and nest site choice on Red-Backed Shrike (Lanius collurio) reproductive success: a comparison of clearcuts and grasslands. In: Yosef R, Lohrer FE (eds) Shrikes of the World-II: Conservation Implementation. Proceedings of the Second International Shrike Symposium, 17–23 March 1996, International Birdwatching Center in Eilat, Israel, pp 74–75
Syrová M, Němec M, Veselý P, Landová E, Fuchs R (2016) Facing a clever predator demands clever responses—red-backed shrikes (Lanius collurio) vs. Eurasian magpies (Pica pica). PLoS ONE 11:e0159432
Takagi M, Abe S (1996) Seasonal change in nest site and nest success of Bull-headed Shrikes. Jap J Ornithol 45:167–174
Tryjanowski P (2002) A long-term comparison of laying date and clutch size in the Red-backed Shrike (Lanius collurio) in Silesia, Southern Poland. Acta Zool Acad Sci Hung 48:101–106
Tryjanowski P, Goławski A (2004) Sex differences in nest defence by the Red-backed Shrike Lanius collurio: effects of offspring age, brood size, and stage of breeding season. J Ethol 22:13–16
Tryjanowski P, Kuzniak S, Diehl B (2000) Does breeding performance of red-backed shrike Lanius collurio depend on nest site selection? Ornis Fenn 77:137–141
Tryjanowski P, Karg MK, Karg J (2003) Diet composition and prey choice by the Red-backed Shrike Lanius collurio in western Poland. Belg J Zool 133:157–162
Tryjanowski P, Golawski A, Kuźniak S, Mokwa T, Antczak M (2007) Disperse or stay? Exceptionally high breeding-site infidelity in the Red-backed Shrike Lanius collurio. Ardea 95:316–320
Tyler JD (1995) Reproductive biology of loggerhead shrikes in southwestern Oklahoma. Proc West Found Vert Zool 6:242–243
UMWM (2020) Structure of ownership and use of land in the Mazowieckie voivodeship. https://geodezja.mazovia.pl/. Accessed 05 Feb 2023
Verhulst S, Nilsson JA (2008) The timing of birds’ breeding seasons: a review of experiments that manipulated timing of breeding. Philos Trans R Soc Lond B Biol Sci 363:399–410
Veselý P, Syrová M, Voháňková M, Havlíček J, Nácarová J, Fuchs R (2022) Cowards or clever guys: An alternative nest defence strategy employed by shrikes against magpies. Anim Cogn 25:307–317
Weggler M (2000) Reproductive consequences of autumnal singing in black redstarts (Phoenicurus ochruros). Auk 117:65–73
Weidinger K (2002) Interactive effects of concealment, parental behaviour and predators on the survival of open passerine nests. J Anim Ecol 71:424–437
Wesołowski T (1986) Card index of nests and broods. Instructions for co-workers. University of Wrocław
Wozna JT, Hromada M, Reeve NF, Szymański P, Zolnierowicz KM, Tobolka M (2017) Patchy versus linear non-cropped habitats in farmland: which is better for nesting success of the Red-backed Shrike Lanius collurio? Bird Study 64:98–103
Yosef R (2000) Nesting ecology of the Loggerhead Shrike (Lanius ludovicianus) in southcentral Florida. Ring 22:111–121
Yosef R, Pinshow B (1988) Nestling transfer in the Northern Shrike (Lanius excubitor). Auk 105:580–581
Zduniak P (2010) Water conditions influence nestling survival in a Hooded Crow Corvus cornix wetland population. J Ornithol 151:45–50
Zduniak P, Czechowski P, Jędro G (2011) The effect of nesting habitat on reproductive output of the Barn Swallow (Hirundo rustica). A comparative study of populations from atypical and typical nesting habitats in western Poland. Belg J Zool 141:38–43
Acknowledgements
We thank Peter Senn for correcting the English. We also thank the two anonymous referees for their numerous critical and useful comments on previous drafts of this paper. This research was supported by the Siedlce University of Natural Sciences and Humanities, Poland (Theme No. 76/20/B financed from a science grant by the Ministry of Education and Science, Poland to AG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Golawski, A., Mroz, E., Golawska, S. et al. Brood survival in the Red-backed Shrike Lanius collurio in eastern Poland. J Ornithol 164, 921–929 (2023). https://doi.org/10.1007/s10336-023-02084-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-023-02084-6