Abstract
Numerous Woodchat Shrike (Lanius senator) populations, and Laniidae in general, are globally declining. A deeper understanding of their breeding ecology, including their nest-site selection and breeding performance, from hatching success to post-fledging survival, could contribute to their conservation. We studied breeding Woodchat Shrikes near the southern edge of their breeding range in Northern Israel, monitoring breeding pairs throughout the breeding season from March to July 2023 in three study areas at the slopes of the Golan Heights in a semi-open shrubland. We collected data on birds’ nesting success, including hatching and fledging success, as well as on breeding pairs’ productivity and post-fledging survival of Woodchat Shrikes. Furthermore, we gathered information on nest-site characteristics. In this part of their range, Woodchat Shrikes breed in relatively high densities, opportunistically selecting nest-sites within the area. Most pairs had at least two brood attempts and up to four were recorded. On average breeding pairs laid 4.63 eggs per brood, with a decreasing brood size later in the season. Breeding performance was not related to the nest-site characteristics with the exception of nest height above the ground, which had a positive influence on nesting success. The breeding performance of Woodchat Shrikes was relatively low compared to other studies, particularly when considering fledging success. Nest predation was likely a predominant cause for nest failures here; however, breeding pairs were able to substantially increase their breeding success with replacement broods. Compared to the low nesting success, the post-fledging survival was relatively high, indicating much higher predation pressure on nests compared to fledglings.
Zusammenfassung
Bruterfolg und Nistplatzwahl des Rotkopfwürgers Lanius senator am Südrand seiner Brutverbreitung
Viele Populationen des Rotkopfwürgers (Lanius senator) und der Laniidae generell sind weltweit rückläufig. Zu ihrer Erhaltung könnte beitragen, ihre Brutökologie, einschließlich der Wahl des Nistplatzes und des Bruterfolgs vom Schlüpfen bis zum Überleben nach dem Ausfliegen, zu verstehen. Wir untersuchten brütende Rotkopfwürger nahe dem südlichen Rand ihres Brutgebietes in Nordisrael und beobachteten die Brutpaare während der gesamten Brutsaison von März bis Juli 2023 in drei Untersuchungsgebieten in einem halboffenen Buschland an den Hängen der Golanhöhen. Wir sammelten Daten zum Bruterfolg der Vögel, einschließlich des Schlüpfens und Ausfliegens, zur Produktivität der Brutpaare, zum Überleben der Rotkopfwürger nach dem Ausfliegen und außerdem noch zu speziellen Merkmalen der Nistplätze. In diesem Teil ihres Verbreitungsgebiets brüten die Rotkopfwürger relativ nah beieinander, wobei sie ihre Nistplätze in dem Areal ganz willkürlich auswählen. Die meisten Paare hatten mindestens zwei Brutversuche, aber es konnten bis zu vier festgestellt werden. Im Schnitt legten die Brutpaare pro Brut 4,63 Eier, wobei deren Anzahl im Verlauf der Saison abnahm. Der Bruterfolg stand in keinem Zusammenhang mit irgendwelchen Merkmalen des Nistplatzes, mit Ausnahme der Nesthöhe über dem Boden, die sich positiv auf den Bruterfolg auswirkte. Im Vergleich mit anderen Untersuchungen war der Bruterfolg der Rotkopfwürger relativ gering, vor allem in Hinblick auf die Anzahl der flügge werdenden Jungvögel. Nesträuber waren hier wahrscheinlich die Hauptursache für die Verluste bei den Bruten; aber die Brutpaare konnten ihren Bruterfolg durch Zweitbruten erheblich steigern. Im Vergleich zum geringen Bruterfolg war die Überlebensrate nach dem Ausfliegen relativ hoch, was auf einen viel höheren Beutedruck auf die Nester als auf die Jungvögel hinweist.
Similar content being viewed by others
Introduction
Declines in bird populations across Europe are largely driven by agricultural intensification and land-use changes since the twentieth century, causing negative population trends especially in birds adapted to open landscapes (Bowler et al. 2021; Rigal et al. 2023). However, spatiotemporal dynamics in bird population differ across Europe (Tryjanowski et al. 2011). These dynamics also depend on birds’ migration strategies; long-distance migrants have exhibited greater decline (Bowler et al. 2021) and generally have lower seasonal breeding performance due to the shorter time for breeding in their annual cycle (Smith and Moore 2005). To implement effective conservation measures averting further population declines, it is crucial to gain knowledge on species’ breeding ecology and environmental requirements (Tryjanowski et al. 2011). In addition to nesting success (nests producing at least one fledgling) and breeding success (pairs producing at least one fledgling), information on breeding performance should also include fledglings’ survival during the period after young birds leave the nest and until they disperse or migrate, referred to as post-fledging survival (Anders and Marshall 2005; Cox et al. 2014). During this post-fledging period, many young rely on parental care, especially for species with short nesting periods (Grüebler and Naef-Daenzer 2010). Even though this period is particularly relevant in species fitness, it is under-studied in most passerines and thus studies quantifying the survival during this period are crucial (Anders and Marshall 2005; Cox et al. 2014).
As many other members of the Laniidae family, Woodchat Shrikes (Lanius senator) are adapted to open landscapes and preferably breed in semi-open, dry grassland habitats with scattered shrubs, but they are also found in agricultural fields with sufficient shrub-like cover (Isenmann and Fradet 1998). Furthermore, most members of the Laniidae family worldwide are declining (Yosef 1994; Lefranc 2022), and so are many European breeding populations of the Woodchat Shrike (Yosef 2008). Reduction and degradation of the Shrikes’ breeding habitat and increased mortality in the breeding grounds and along their migration routes have caused population declines, especially in Northwest Europe (Yosef and Tryjanowski 2000; Yosef 2008; Lefranc 2022). Woodchat Shrikes prey almost exclusively on large invertebrates, requiring prey-rich habitats with certain habitat structures for their “sit-and-wait” hunting strategy, but most suitable habitat types are shrinking throughout Europe (Cramp et al. 1993; Yosef 2008). Although their northern breeding distribution range is contracting, Woodchat Shrikes are still common in most of their southern breeding areas (Yosef 2008). Thus, despite their decline, they are not considered to be globally threatened (BirdLife International 2023).
We studied Woodchat Shrikes (subspecies: L. s. niloticus) in Northern Israel, along the slopes of the Golan Heights. This is one of the southernmost breeding locations of this species. Consequently, L. s. niloticus have a shorter migration route to their Northeast African wintering grounds, resulting in a longer breeding season compared to the European subspecies (Cramp et al. 1993). We studied the breeding performance of Woodchat Shrikes during different stages of their breeding season and estimated the survival of their eggs, nestlings, and fledglings. This study also investigated the nest-site selection to understand how it affected the birds’ breeding performance. Here, we investigated: (1) nest-site selection and breeding densities of Woodchat Shrikes; (2) if breeding performance was affected by nest-site selection; and (3) post-fledging survival rate and breeding productivity. This study enhances our understanding of Woodchat Shrike breeding ecology, allowing us to determine which nest-site characteristics contribute to successful reproduction, in a population near the southern edge of the species’ breeding distribution range.
Materials and methods
Data collection
During the breeding season from March to July 2023, we monitored Woodchat Shrikes in three study areas, of 22–29 ha, that consist of semi-open shrubland with extensive grazing pressure: Ein Tina (33°04′56.0"N, 35°38′45.1"E), Nahal Neshef (33°05′38.0"N, 35°38′46.5"E), and Nahal Orvim (33°09′02.5"N, 35°39′30.6"E). Each study area was visited weekly searching for nests to record the number of eggs or nestlings as well as to collect information on nest-site characteristics, i.e., nest height above the ground, nest concealment, and nest-supporting shrub species. The nest concealment categories (see Fig. 1c, d, e) were defined evaluating the nests’ visibility from two points, at a 90-degree angle to one another, when standing two meters in front of the nest-supporting shrub. By revisiting the nest-sites weekly, we monitored the Shrikes’ breeding performance. To minimize nest failures due to human disturbance, specific nests that contained eggs were only controlled again after two weeks (Shirihai 1996; Tryjanowski and Kuźniak 1999). From approximately 8-days of age, nestlings were ringed with unique combinations of coded metal rings and uncoded colour rings. This enabled individual identification of the young during the regular (i.e., at least twice a week) resighting visits of pairs with fledglings during the post-fledging period, using binoculars and a spotting scope. Meteorological data were collected from the Israeli Metrological Service's weather station of in Kfar Blum, about eight km northwest of the study areas (Israel Meteorological Service Meteorological Database 2023).
Images of a the predominant semi-open shrubland habitat at the study areas and b a Woodchat Shrike (Lanius senator) nest. Three nest concealment categories were defined: c densely concealed nests, which cannot be easily seen, d intermediately concealed nests, which are only partially visible, and e open nests, that are completely visible. The light grey arrow indicates the nest location within the shrub
Data analysis
We calculated the weekly breeding pair density per ha for each study area from the number of active breeding pairs (i.e., incubating or with nestlings), based on field observations and estimated egg-laying dates. For nests found with only the first egg laid or with nestlings (n = 29), we estimated the one- to two-day precise egg-laying date retrospectively, after identifying nestling ages in days post-hatching, following the description for aging Red-backed Shrike (Lanius collurio) nestlings by Van den Burg et al. (2011). For the remaining nests (n = 32), we estimated the two- to 12-days precise egg-laying date from the number of eggs in combination with field observations or the age of the fledglings, identified from morphological characteristics. To calculate the egg-laying date, we used the mean incubation (15 days) and nestling periods (16 days) described in other studies of Woodchat Shrikes (Ullrich 1971; Shirihai 1996; Bechet et al. 1998; Isenmann and Fradet 1998; Nikolov 2005; Yosef 2008). Using recent aerial images (0.6 m resolution) in ArcGIS 10.6.1 (Esri Inc. 2023), we performed spatial analyses calculating additional nest-site characteristics. We identified the distance to the nearest neighbouring active nest, the position of the nest-supporting shrub in relation to other shrubs, the distance between nests used for different brood attempts by the same pair and the number of shrubs in the territory. We used a 1.5 ha large buffer area around each nest as territory, since this territory size was described in other studies (Yosef 2008) and fits to the density and mean distance between nests in the present study. To calculate the mean clutch size, we included nests with at least two eggs, because two to seven eggs were found in the nests with information on complete clutch, as confirmed from a second nest visit (see SI1). We tested if the clutch size varied in relation to the egg-laying date, using a Spearman’s Rank Correlation, and between the number of brood attempts, using a Kruskal–Wallis test and Dunn´s post-hoc test. We tested the re-nesting distance in relation to the distance to the nearest neighbouring active nests using a Spearman’s Rank Correlation. Additionally, we tested if the probability for a replacement brood depends on the egg-laying date of the failed brood attempt using a binomial Generalized Linear Model (GLM). Brood attempts were identified based on observed behaviours and movements of the breeding pairs during the weekly site visits. Since nearly half of the breeding pairs had at least one colour-ringed adult (i.e., 15 out of 31 pairs), we were able to follow relocation movements during replacement broods.
We calculated various variables of the Woodchat Shrikes’ breeding performance: a brood attempt was defined if at least one egg was laid. For these nests we calculated the hatching success (percentage of eggs that hatched from laid eggs), fledging success (percentage of fledged young from hatched nestlings) and nesting success (percentage of nests with at least one fledgling). For breeding pairs, we calculated the breeding success (percentage of pairs with at least one fledgling) and productivity (number of fledglings, both for all and only for successful pairs).
To test whether nest-site selection influenced nesting success, we ran a binomial Generalized Linear Mixed Model (GLMM) with the breeding pair as random factor and different variables related to the nest-site characteristics: study area (Ein Tina, Nahal Neshef or Nahal Orvim), nest concealment (dense, intermediate or open), nest height above the ground, shrub species (Ziziphus spina-christi, Ziziphus lotus, or Pistacia atlantica), shrub position (solitary or in a shrub group), weekly breeding pair density while the nest was active, distance to nearest neighbouring nest, and number of shrubs in the pair’s territory (n = 62 nests). The different models were ranked according to their AIC value, and the best fitted model with a subset of variables (i.e., nest height, nest concealment, and shrub species, see SI6) was selected as the final model. Furthermore, we performed binomial GLMM, with the breeding pair as random factor, to test for a possible correlation between nesting success and clutch size (n = 52), estimated egg-laying date (n = 61), and different brood attempts (n = 60), respectively. We used binomial and poisson GLMMs, with the breeding pair as random factor, to test how breeding success and productivity of breeding pairs (n = 31), respectively, varied with the different nest-site selection factors: study area, mean nest height above the ground, mean weekly breeding pair density, mean distances to nearest neighbour, and number of shrubs in the territory.
To study post-fledging survival, we calculated the young survival over the study period (8–49 days after hatching) as a product of the daily apparent survival rate (i.e., probability that an individual is alive) for each day. We estimated the daily apparent survival rate and the probability to observe an individual for each day running a “young survival for marked adults” model, an extension to the Cormack-Jolly-Seber model with resighting data of the colour-ringed young of each breeding pair (Lukacs and Dreitz 2008), in the program MARK 9.0 (White 2021). To test if the daily apparent survival was related to their age, we ran a Spearman’s Rank Correlation.
All statistical analyses were performed in RStudio using R version 4.3.1 (R Core Team 2023). All tests were two-tailed with α of 0.05.
Results
Nest-site selection, clutch size, and breeding phenology
Woodchat Shrikes breed in densities with an average of 0.32 active breeding pairs per ha (SD = 0.10) and with 130.65 m (SD = 49.84) between active nests. The number of shrubs in their territories varied substantially, ranging from 2 to 79 shrubs (mean = 39.16; SD = 18.71). The birds built their nests mostly in Ziziphus spina-christi shrubs (87.1%). The majority (54.8%) of nest-supporting shrubs stood solitarily, and the nest was often openly exposed (43.6%); only 17.7% of the nests were hidden in dense shrubs. The nests were 1.17–42 m above the ground level, with a mean height of 1.93 m (SD = 0.65).
The mean clutch size was 4.63 (SD = 1.25), ranging from 2 to 7 eggs (Fig. 2). Many (77.8%) breeding pairs had at least one additional brood attempt, but up to four brood attempts were recorded. The clutch size was significantly lower in the third compared to the first (p < 0.001, Z = 4.01) and second broods (p < 0.05, Z = 2.89, SI2) and decreased significantly with later egg-laying date (p < 0.01, rs = – 0.59, SI3). On average, the re-nesting distance between brood attempts was 69.7 m (SD = 45.18, range: 10.7 to 235.7 m, n = 32). The re-nesting distance was not significantly correlated with the distance to the nearest neighbouring breeding pair (SI4). More than half of the failed nests (65.1%) had a replacement brood. The probability for a replacement brood was significantly lower (p < 0.01, b = − 0.13, n = 43, SI5) with later egg-laying dates and brood attempts that failed after the 6th of June had no replacement broods.
Woodchat Shrikes started to breed, i.e., laid eggs of their first brood attempt, on average on the 17th of April (SD = 8.22, n = 25), but this varied between breeding pairs, with a total range of 36 days between the earliest pair (end of March) and the last (beginning of May). The breeding phenology (Fig. 3) had two peaks, of pairs either incubating or with nestlings, in the beginning and at the end of May. From the end of April, the first breeding pairs were already observed with their fledglings.
Breeding success, productivity, and post-fledging survival
Of the brood attempts with at least one laid egg, 55.4% hatched successfully and 29.8% of the hatched young also survived until fledging. Consequently, the overall nesting success was 21.9%. However, due to the replacement broods of breeding pairs with failed attempts, the breeding success was increased to 39.3%. Most of the failed nests (60%) were found empty but undestroyed, as after predation due to avian or reptile nest predators (Yosef 2000). None had dead nestlings inside, as it is the case after nest failure due to bad weather events (Mirkov 1984). Days with over five mm rainfall recorded after the earliest egg-laying date were rare, with a single event on the 29th of April (7.9 mm) (Israel Meterological Service Meterological Database 2023). On average, the breeding pairs produced 1.19 fledglings when considering all pairs, and 3.08 fledglings when considering only the successful pairs (Table 1).
The GLMM that included the variable nest height (together with nest concealment and shrub species) was the best fitted model according to its AIC (61.00) with a weight of 0.089. The second-best model had an AIC of 61.36 (SI6). Nesting success was significantly and positively (p < 0.05, b = 1.70, SI7) related to the nest height. All other variables related to the nest-site selection were not significant in models of nesting success, breeding success, or productivity (SI7, SI11–SI12). The clutch size, egg-laying date, and different brood attempts also did not affect nesting success (SI8–SI10).
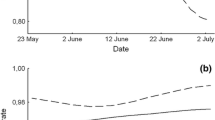
Using field observations of colour-ringed young, we found that fledglings dispersed from their natal nest-site 16–41 days after fledging. Until then, they remained together in family groups under parental care. Over this period, young had a mean survival rate of 0.739 (SD = 0.33). The daily apparent survival of the young increased significantly (p < 0.001, rs = 1, Fig. 4, SI13) with their age.
Estimate of the daily apparent survival rate (black line) with the standard error (grey area) of 8- to 49-day-old Woodchat Shrikes (Lanius senator) increased significantly (p > 0.001, rs = 1, SI13) with age (i.e., days after hatching). The data is from seven breeding pairs with colour-ringed young (n = 27 young). The vertical, red, dashed line indicates 16-days of age, post-hatching, when nestlings usually fledge (Ullrich 1971; Shirihai 1996; Bechet et al. 1998; Isenmann and Fradet 1998; Nikolov 2005; Yosef 2008)
Discussion
Nest-site selection and breeding ecology
Woodchat Shrikes at our study area breed in their generally preferred habitat type, a semi-open shrubland (Isenmann and Fradet 1998; Yosef 2008). It provides all required habitat structures (Yosef 2008), and the birds show some flexibility in relation to shrub density preference, a habitat attribute which varied substantially between territories. As also found in other studies, Woodchat Shrikes are largely opportunistic in their choice of nest support, selecting the most common shrub species (Isenmann and Fradet 1998), which, in the present study, was the Ziziphus spina-christi, a thorny but loosely vegetated shrub species. Thus, most nests in our study area were poorly concealed. Nest height varies widely within and between studies (Cramp et al. 1993). Compared to European study areas (Cramp et al. 1993), nests in the present study were rather low (mean height of 1.93 m above the ground), similar to those reported from other Mediterranean populations (Yosef 2008). Southern populations, like in the present study, breed in more clustered structures, with smaller territory sizes, than in Central Europe (Yosef 2008). The mean distance of 130.65 m between active nests in this study, also indicates a small territory size like the 1.5 ha described for Woodchat Shrikes in Georgia (Yosef 2008). The relatively high breeding density of 0.32 pairs per ha is slightly higher than 0.18/ha in Algeria (Moali et al. 1997) and similar to 0.3/ha in Morocco (Brosset 1961), but not as high as the exceptionally dense breeding populations in Spain with 1.03 pairs per ha (Rehsteiner 2012) or 1.65/ha in Bulgaria (Karaivanov 2005). Nevertheless, to calculate the density, we only included breeding pairs incubating or with nestlings, while other studies used data from all the present adults with a territory. Using that classification would also include family groups with fledglings (see Fig. 3), resulting in a higher density of up to 0.41 breeding pairs per ha.
The mean clutch size of 4.63 eggs was slightly lower than those described in other studies, which only included clutches with at least three eggs (Ullrich 1971; Bechet et al. 1998; Isenmann and Fradet 1998; Nikolov 2005; Yosef 2008). However, the complete clutches found in the present study ranged from two to seven eggs (SI1), indicating a smaller minimum clutch size. Woodchat Shrikes in the present study had a decreasing clutch size both with each replacement clutch and with the progression of the breeding season, a pattern commonly observed in many Shrike species (Ullrich 1971; Isenmann and Fradet 1998; Kristin et al. 2000; Antczak et al. 2009). Most breeding pairs had more than one brood attempt, which is usual in Middle Eastern and North African breeding population (Cramp et al. 1993; Yosef 2008). Interestingly, one colour-ringed female laid eggs in four brood attempts, an exceptionally high number that was not described before for Woodchat Shrikes. The Shrikes had a replacement brood after more than half of the failed brood attempts recorded, even more often than the Woodchat Shrikes in Mediterranean France (Isenmann and Fradet 1998). Replacement broods are costly, and for Red-backed Shrikes, the size and probability of a replacement clutch depends on the stage of the failed brood (Antczak et al. 2009). The present study did not have sufficiently large sample size on the stage of the nest failure to explicitly test this pattern. Nevertheless, this information was likely important in the decision-making process of the breeding pairs, as the probability for a replacement brood decreased significantly over the breeding season and failed broods with eggs laid after the 6th of June were not replaced anymore. The majority of Woodchat Shrikes in our study re-nested within the 1.5 ha territory around their previous nest-site, as was also observed for Red-backed Shrikes (Antczak et al. 2009). Some Woodchat Shrikes, however, were found to re-nest outside their previous territory, up to 235.7 m away, indicating some spatial flexibility. However, the re-nesting distance seems to be independent of the distance to the pair’s nearest neighbour.
In line with the description in the literature (Shirihai 1996), our field observations suggest that the first birds of the L. s. niloticus subspecies arrive from migration to their breeding grounds as early as March. Their egg-laying largely began in April, slightly later and more variably than described previously for breeding populations in Israel, yet it was still substantially earlier than in Central Europe (Yosef 2008). In Central Europe, the Woodchat Shrikes must start their nest building considerably faster after arriving at the breeding grounds (Ullrich 1971), indicating important time-constraints during their shorter breeding season due to longer migration distances compared to the L. s. niloticus subspecies (Cramp et al. 1993). This might also be supported by their summer moult pattern, where the nominate subspecies does not replace any flight feathers (Svensson 1992), while adult L. s. niloticus Woodchat Shrikes only retain a few secondaries from their extensive split moult (Kiat and Izhaki 2022).
The importance of nest-site selection for breeding success
Woodchat Shrikes with nests higher above the ground had a significantly higher nesting success in this study, a pattern previously observed across several passerine species (Li and Martin 1991), including Loggerhead Shrikes (Lanius ludovicianus) (Nur et al. 2004). Higher nests might be safer from ground predators, like some mammals and reptiles, since Great Grey Shrikes (Lanius excubitor) increased their nest height after human disturbance (Antczak et al. 2005). However, some studies on Woodchat, Red-backed- and Great Grey Shrikes found little or no evidence for the importance of nest height on nesting success (Yosef 1992; Isenmann and Fradet 1998; Tryjanowski et al. 2000). In the present study, the GLMM including the variable nest height had relatively low support, with a weight of only 0.089, and several other models were within ∆AIC less than two (SI6), the widely accepted “substantial level of empirical support” threshold (Burnham and Anderson 2002).
No other nest-site characteristics showed importance for Woodchat Shrike breeding performance, i.e., nesting success, breeding success or productivity, in support of other studies (Isenmann and Fradet 1998). Similarly, for a variety of passerines, including Shrikes, there was no effect of nest concealment on breeding success (Isenmann and Fradet 1998; Campos et al. 2011; Golawski et al. 2023). In studies that also included thornless deciduous shrubs, the Iberian Grey Shrike (Lanius meridionalis) and Red-backed Shrike both had higher success in the thorny than in thornless shrubs (Tryjanowski et al. 2000; Campos et al. 2011). In our study, all three nests in the thornless Pistacia atlantica were predated, but the low sample size did not allow for a statistical comparison to the 59 nests found in thorny shrubs. The properties of thorny shrubs as nest sites seem to increase the effectiveness of nest defence in Red-backed Shrikes, especially during the early nestling stage (Tryjanowski et al. 2000; Golawski and Mitrus 2008). However, higher visibility from the nest might be beneficial for adults who can start warning others and defending their nest earlier than from highly concealed nests (Golawski et al. 2023). While studies on the closely related Red-backed Shrike show the importance of a parent’s aggressiveness for the breeding success (Golawski and Mitrus 2008), corresponding studies in Woodchat Shrikes are lacking. Interestingly, aggressive behaviour against potential avian nest predators, i.e., Hooded Crows (Corvus cornix) and Eurasian Jays (Garrulus glandarius), were frequently observed at the study areas (personal observations), stressing the need for further studies on the importance of aggressiveness for nesting success of Woodchat Shrikes. In previous studies, general breeding habitat type affected breeding performance in Red-backed Shrikes (Bloche et al. 2023), possibly because predation danger is related to the breeding and surrounding habitat types (Matyjasiak 1995; Söderström et al. 1998). In our study the habitat type was essentially the same in all the study areas, but in further studies, breeding pairs from other habitat types should be compared, e.g., Woodchat Shrikes breeding higher up in the Golan Heights or even at the nearby Mt. Hermon in mountainous habitats between 1000 and 1600 m above sea level (Shirihai 1996).
Woodchat Shrikes in the present study had a relatively low nesting success compared to other studies (Bechet et al. 1998; Isenmann and Fradet 1998; Nikolov 2005). Specifically, fledging success was severely lower than in other studies, while the hatching success seems to be similar (Ullrich 1971; Hudec and Černý 1983; Nikolov 2005). This difference between hatching and fledging success indicates that most nest failures were during the nestling stage. During this period, the frequent nest visits by feeding adults might make the nests more conspicuous to predators (Martin et al. 2000). Golawski et al. (2023) also found lower survival during the nestling stage, while Nikolov (2005) found that most nesting attempts of Woodchat Shrikes failed before hatching.
The results of our one-year study should be treated with caution, as Shrike breeding populations can fluctuate strongly between years (Ullrich 1971, 1987; Bloche et al. 2023; Golawski et al. 2023). Nevertheless, Bechet et al. (1998) did not find fluctuation of the predation rate on Woodchat Shrikes nests. The Woodchat Shrikes in our study were able to increase the originally low nesting success with replacement broods to a much higher success rate of 39.3% for the pairs’ breeding success. This stresses the importance of replacement broods for the long-term maintenance of Shrike populations. To have the chance for re-nesting after a failed brood attempt, Red-backed Shrikes evolved to start nesting as early as possible (Antczak et al. 2009). While the European populations of Woodchat Shrikes only arrive at the breeding grounds in mid-May (Cramp et al. 1993), the L. s. niloticus subspecies might already start its second brood in May. This is a critical advantage of short-distance migration, also demonstrated through generally higher annual fecundity (Bruderer and Salewski 2009) and seasonal breeding performance (Smith and Moore 2005) of short-distance migrants, compared to long-distance migrants.
Nest predation likely was the predominant cause for nest failures in this study, as it was for Woodchat Shrikes in Bulgaria (Nikolov 2005) and Mediterranean France (Bechet et al. 1998). This was also documented for other Shrike species, including Lesser Grey Shrikes (Lanius minor) in Slovakia (Kristin et al. 2000) and Great Grey Shrikes in Israel (Yosef 1992, 1993). While inclement weather, i.e., cold and rainy periods, is a common cause for nest failures in Central Europe (Ullrich 1971), it was probably not as relevant in our study area. The single rain event documented during this study was within the period in which only six out of 38 nest failures occurred, and no nest was found with dead nestlings, as is typically the case after inclement weather (Mirkov 1984). Other studies of Mediterranean breeding populations mention several potential nest predators of Woodchat Shrikes (Bechet et al. 1998). Avian nest predators were commonly observed at our study area (personal observations). Moreover, several reptiles, like snakes and the European Glass Lizard (Ophisaurus apodus), as well as mammals, are potential nest predators in the Mediterranean shrubland (Ben-David et al. 2019). Most failed nests here were found empty and undestroyed, which, according to Yosef (2000), indicates predation by avian or reptile nest predators and not mammals. The significant effect of nest height on nesting success might indicate the importance of ground predators, i.e., reptiles. However, further studies are needed to identify and quantify the specific nest predators of Woodchat Shrikes in Israel, since predation seems to greatly decrease their nesting success.
Breeding productivity and post-fledging survival
The Woodchat Shrikes had relatively low breeding productivity due to the low nesting success. While the productivity of successful pairs was only slightly lower than in other studies, the productivity of all breeding pairs was severely below what was found in comparable studies (Ullrich 1971; Hudec and Černý 1983); however, the survival rate of the young after fledging was substantially higher. The post-fledging survival rate of 0.739 for young Woodchat Shrikes is similar to results for Red-backed Shrikes (0.73) (Bloche et al. 2023) and Loggerhead Shrikes (0.76) (Imlay et al. 2010). The daily survival rate of young Woodchat Shrikes increased over the post-fledging period (Fig. 4) along with increasing independence from parental care (Nikolov and Hristova 2007; Bloche et al. 2023). This pattern is commonly observed across passerines, including Shrikes (Yackel Adams et al. 2001; Cox et al. 2014; Bloche et al. 2023). Even though parental care during this period is crucial for the survival of the young (Nikolov and Hristova 2007), we found large variation in the length of the period when fledglings stay in family groups at their natal nest-site until dispersal. While some only stayed for just over two weeks after fledging, as described by Shirihai et al. (1996), fledglings in other breeding pairs stayed for up to six weeks, as described in other studies (Nikolov and Hristova 2007; Yosef 2008). Further studies on the behaviour, movements and dispersal of young Woodchat Shrikes are needed to better understand the causes and consequences of this variation.
Conclusion
Aside from the nest height above ground, no other nest-site characteristics were related to Woodchat Shrikes breeding performance in this study. Similar to comparable studies, nesting success substantially decreased, as a result of predation, before young fledged (Isenmann and Fradet 1998). This study stresses the importance of replacement broods for the overall breeding success in Shrikes (Antczak et al. 2009) and the advantages of having fewer breeding time-constraints in short-distance migrants (Smith and Moore 2005). When developing conservation measures in different regions, the importance of replacement broods and differences in breeding time-constraints between different populations needs to be considered. Furthermore, the high breeding densities at the study area indicate the importance of semi-natural habitat types, i.e., semi-open shrubland with extensive grazing pressure. Since nest-site selection might be less important for breeding performance than the local abundance of predators (Golawski et al. 2023), it is crucial to identify specific nest predators of Woodchat Shrikes. Additionally, we highlight important knowledge gaps regarding the length of the post-fledging period under parental care as well as post-fledging dispersal behaviour and movement. It is crucial to close these knowledge gaps to create species-specific conservation measures for Woodchat Shrikes in Israel, near the southern edge of their breeding distribution.
Data availability
The main data from the figures and results are public available on figshare.com via the following link: https://figshare.com/projects/Breeding_performance_and_nestsite_selection_of_Woodchat_Shrikes_Lanius_senator_near_the_southern_edge_of_their_breeding_distribution/198508.
References
Anders AD, Marshall MR (2005) Increasing the accuracy of productivity and survival estimates in assessing landbird population status. Biol Conserv 19:66–74. https://doi.org/10.1111/j.1523-1739.2005.00543.x
Antczak M, Hromada M, Tryjanowski P (2005) Research activity induces change in nest position of the Great Grey Shrike Lanius excubitor. Ornis Fenn 82:20–25
Antczak M, Golawski A, Kuzniak S, Tryjanowski P (2009) Costly replacement: How do different stages of nest failure affect clutch replacement in the Red-backed Shrikes Lanius collurio? Ethol Ecol Evol 21:127–136. https://doi.org/10.1080/08927014.2009.9522501
Bechet A, Isenmann P, Gaudin R (1998) Nest predation, temporal and spatial breeding strategy in the Woodchat Shrike Lanius senator in Mediterranean France. Acta Oecol 19:81–87. https://doi.org/10.1016/S1146-609X(98)80011-8
Ben-David A, Shamon H, Izhaki I, Efronny R, Maor R, Dayan T (2019) Increased songbird nest depredation due to Aleppo Pine (Pinus halepensis) encroachment in Mediterranean shrubland. BMC Ecol. https://doi.org/10.1186/s12898-019-0270-8
BirdLife International (2023) Species factsheet: Lanius senator. http://datazone.birdlife.org/species/factsheet/woodchat-shrike-lanius-senator. Accessed 17 September 2023.
Bloche DAF, Thorup K, Olsen K, Larsen PE, Strange K-E, Tøttrup AP (2023) Breeding biology of Red-backed Shrikes (Lanius collurio): Distribution, performance and post-fledging survival in Denmark. Ornis Fenn 100:69–83. https://doi.org/10.5181/of.124729
Bowler D, Richter RL, Eskildsen D, Kamp J, Moshøj CM, Reif J, Strebel N, Trautmann S, Voříšek P (2021) Geographic variation in the population trends of common breeding birds across Central Europe. Basic Appl Ecol 56:72–84. https://doi.org/10.1016/j.baae.2021.07.004
Brosset A (1961) Ecologie des Oiseaux du Maroc Oriental. Travaux de l'Institut Scientifique Cherifien. Série Zoologie 22
Bruderer B, Salewski V (2009) Lower annual fecundity in long-distance migrants than in less migratory birds of temperate Europe. J Ornithol 150:281–286. https://doi.org/10.1007/s10336-008-0348-0
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Campos F, Santamara T, Gutirrez-Corchero F, Ngeles Hernndez M, Mas P (2011) Breeding success of Southern Grey Shrikes Lanius meridionalis in agricultural areas: The influence of nest site characteristics. Acta Ornithol 46:29–36. https://doi.org/10.3161/000164511X589884
R Core Team (2023) R 4.3.1: A Language and Environment for Statistical Computing https://cran.rstudio.com/. Accessed 10 September 2023
Cox WA, Thompson FR, Cox AS, Faaborg J (2014) Post-fledging survival in passerine birds and the value of post-fledging studies to conservation. J Wildl Manag 78:183–193. https://doi.org/10.1002/jwmg.670
Cramp S, Perrins CM, Brooks DJ et al (1993) Handbook of the Birds of Europe the Middle East and North Africa - The Birds of the Western Palearctic. Oxf Univ Press, New York
Golawski A, Mitrus C (2008) What is more important nest site concealment or aggressive behaviour of Red-backed Shrike, Lanius collurio. Folia Zool Brno 57:403–410
Golawski A, Mroz E, Golawska S, Parapura A, Zduniak P (2023) Brood survival in the Red-backed Shrike Lanius collurio in eastern Poland. J Ornithology 164:921–929. https://doi.org/10.1007/s10336-023-02084-6
Grüebler MU, Naef-Daenzer B (2010) Survival benefits of post-fledging care: experimental approach to a critical part of avian reproductive strategies. J Anim Ecol 79:334–341. https://doi.org/10.1111/j.1365-2656.2009.01650.x
Hudec K, Černý W (1983) Fauna ČSSR Ptáci-Aves III/1. Academia, Prague
Imlay TI, Crowley JF, Argue AM, Steiner JC, Norris DR, Stutchbury BJM (2010) Survival, dispersal and early migration movements of captive-bred juvenile Eastern Loggerhead Shrikes (Lanius ludovicianus migrans). Biol Conserv 143:2578–2582. https://doi.org/10.1016/j.biocon.2010.06.027
Esri Inc. (2023) ArcGIS Desktop 10.6.1: ArcMap and ArcCatalog https://www.esri.com. Accessed 10 September 2023
Isenmann P, Fradet G (1998) Nest site, laying period, and breeding success of the Woodchat Shrike (Lanius senator) in Mediterranean France. J Ornithology 139:49–54. https://doi.org/10.1007/BF01651315
Israel Meteorological Service Meteorological Database. https://ims.gov.il/en/data_gov. Accessed 17 September 2023
Karaivanov N (2005) Breeding Bird Communities in Cermes Oak Formations (Quercus coccifera L.) in Bulgaria. In: Proceedings of the Balkan Scientific Conference of Biology. Plovdiv, pp 404–408.
Kiat Y, Izhaki I (2022) Split moult: A response to time constraints during the main moult period. Ibis 165:691–696. https://doi.org/10.1111/ibi.13158
Kristin A, Hoi H, Valera F, Hoi C (2000) Breeding biology and breeding success of the lesser Grey Shrike Lanius minor in a stable and dense population. Ibis 142:305–311. https://doi.org/10.1111/j.1474-919x.2000.tb04870.x
Lefranc N (2022) Shrikes of the World. Bloomsbury, London
Li P, Martin TE (1991) Nest-site selection and nesting success of cavity-nesting birds in high elevation forest drainages. Auk 108:405–418. https://doi.org/10.1093/auk/108.2.405
Lukacs P, Dreitz V (2008) Young survival from marked adults. In: Cooch E (ed) Program MARK, A gentle introduction http://www.phidot.org/software/mark/docs/book. Accessed 10 September 2023
Martin TE, Scott J, Menge C (2000) Nest predation increases with parental activity: separating nest site and parental activity effects. Proc Royal Soc B 267:2287–2293. https://doi.org/10.1098/rspb.2000.1281
Matyjasiak P (1995) Breeding ecology of Red-backed Shrike (Lanius collurio) in Poland. Proc West Found Vertebr Zool 6:228–234
Mirkov I (1984) Breeding of the Woodchat Shrike (Lanius senator L.) in the region of Sofia. Ornithol Inf Bull 15:70–74
Moali A, Ait Sidhoum D, Isenmann P (1997) Quelques Données Sur la Reproduction de la Pie-Grièche à Tête Rousse Lanius Senator en Algérie. Alauda, Dijon. 65:205–207
Nikolov BP (2005) Reproductive success of the Woodchat Shrike (Lanius senator) in Western Bulgaria. Ornis Fenn 82:73–80
Nikolov BP, Hristova IP (2007) Time-activity budgets of juvenile Woodchat Shrikes Lanius senator during the post-fledging period. Ardea 95:235–241. https://doi.org/10.5253/078.095.0206
Nur N, Holmes AL, Geupel GR (2004) Use of survival time analysis to analyze nesting success in birds: an example using Loggerhead Shrikes. Condor 106:457–471. https://doi.org/10.1093/condor/106.3.457
Rehsteiner U (2012) Bestandsentwicklung des rotkopfwürgers Lanius senator in einem steineichenbestand in Spanien über 15 Jahre. Ornithol Beobachter 109:1–8
Rigal S, Dakos V, Alonso H, Auniņš A, Benkő Z, Brotons L, Chodkiewicz T, Chylarecki P, de Carli E, del Moral JC, Domşa C, Escandell V, Fontaine B, Foppen R, Gregory R, Harris S, Herrando S, Husby M, Ieronymidou C, Jiguet F, Kennedy J, Klvaňová A, Kmecl P, Kuczyński L, Kurlavičius P, Kålås JA, Lehikoinen A, Lindström Å, Lorrillière R, Moshøj C, Nellis R, Noble D, Palm Eskildsen D, Paquet JY, Pélissié M, Pladevall C, Portolou D, Reif J, Schmid H, Seaman B, Szabo ZD, Szép T, Florenzano GT, Teufelbauer N, Trautmann S, van Turnhout C, Vermouzek Z, Vikstrøm T, Voříšek P, Weiserbs A, Devictor V (2023) Farmland practices are driving bird population decline across Europe. Proc Natl Acad Sci USA 120:1–9. https://doi.org/10.1073/pnas.2216573120
Shirihai H (1996) The Birds of Israel. Academic Press, London
Smith RJ, Moore FR (2005) Arrival timing and seasonal reproductive performance in a long-distance migratory landbird. Behav Ecol Sociobiol 57:231–239. https://doi.org/10.1007/s00265-004-0855-9
Söderström B, Pärt T, Rydén J (1998) Nest predator faunas and nest predation risk on ground and shrub nests at forest ecotones: an experiment and a review. Oecologia 117:108–118. https://doi.org/10.1007/s004420050638
Svensson L (1992) Identification Guide to European Passerines, 4th edn. Stockholm
Tryjanowski P, Kuźniak S (1999) Effect of research activity on the success of Red-backed Shrike Lanius collurio nests. Ornis Fenn 76:41–43
Tryjanowski P, Kuźniak S, Diehl B (2000) Does breeding performance of Red-backed Shrike Lanius collurio depend on nest site selection? Ornis Fenn 77:137–141
Tryjanowski P, Hartel T, Báldi A, Szymański P, Tobolka M, Herzon I, Goławski A, Konvička M, Hromada M, Jerzak L, Kujawa K, Lenda M, Orłowski G, Panek M, Skórka P, Sparks TH, Tworek S, Wuczyński A, Żmihorski M (2011) Conservation of farmland birds faces different challenges in Western and Central-Eastern Europe. Acta Ornithol 46:1–12. https://doi.org/10.3161/000164511X589857
Ullrich B (1971) Untersuchungen zur ethologie und ökologie des rotkopfwürgers (Lanius senator) in südwestdeutschland im vergleich zu raubwürger (L. excubitor), schwarzstirnwürger (L. minor) und neuntöter (L. collurio). Vogelwarte 26:1–77
Ullrich B (1987) Beringungsergebnisse aus einer brutpopulation des rotkopfwürgers (Lanius senator) im mittleren albvorland, Kreis Göppingen und Esslingen. Ornithol Jahresh Baden-Württ 3:107–112
Van den Burg A, Nijssen M, Geertsma M, Waasdorp S, Van Nieuwenhuyse D (2011) De Grauwe Klauwier—Ambassadeur voor Natuurherstel. KNNV.
White GC (2021) MARK 9.0: Mark and recapture parameter estimation https://www.cnr.colostate.edu/~gwhite/mark/mark.htm. Accessed 01 September 2021
Yackel Adams AA, Skagen SK, Adams RD (2001) Movements and survival of lark bunting fledglings. Condor 103:643–647. https://doi.org/10.1093/condor/103.3.643
Yosef R (1992) From nest building to fledging of young in Great Grey Shrikes (Lanius excubitor) at Sede Boqer, Israel. J Ornithol 133:279–285. https://doi.org/10.1007/BF01645637
Yosef R (1993) Effects of Little Owl predation on Northern Shrike postfledging success. Auk 110:396–398
Yosef R (1994) Conservation commentary: evaluation of the global decline in the true shrikes (Family Laniidae). Auk 111:228–233. https://doi.org/10.2307/4088532
Yosef R (2000) The Mayfield method for standardisation of shrike breeding studies: The case of the Loggerhead Shrike (Lanius ludovicianus) in south-central Florida. The Ring 22:111–121
Yosef R (2008) Family Laniidae (Shrikes). In: del Hoyo J, Elliott A, Christie D (eds) Handbook of the Birds of the World. Lynx Edicions, Barcelona, pp 732–798
Yosef R, Tryjanowski P (2000) Phenology and biometric measurements of migratory Woodchat Shrike (Lanius senator) at Eilat, Israel. The Ring 22:213–217
Acknowledgements
We are grateful to Angelia Ellvin and Daniela Zinßmeister for their assistance in preparing and conducting the fieldwork and to the Israeli Meteorological Service for providing the meteorological data.
Funding
Open access funding provided by University of Haifa.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Daniel A.F. Bloche. The first draft of the manuscript was written by Daniel A.F. Bloche and Nir Sapir commented on previous versions of the manuscript. Both authors read and approved the final manuscript. No funding was received for conducting this study. This is an observational study without invasive procedures. A permit from the Israel Nature and Parks Authority (No. 2023–43275) was granted for undertaking this study.
Corresponding author
Additional information
Communicated by F. Bairlein.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bloche, D.A.F., Sapir, N. Breeding performance and nest-site selection of Woodchat Shrikes Lanius senator near the southern edge of their breeding distribution. J Ornithol (2024). https://doi.org/10.1007/s10336-024-02157-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10336-024-02157-0