Abstract
Among the 28 reporting and data systems (RADS) available in the literature, we identified 15 RADS that can be used in Magnetic Resonance Imaging (MRI). Performing examinations without using gadolinium-based contrast agents (GBCA) has benefits, but GBCA administration is often required to achieve an early and accurate diagnosis. The aim of the present review is to summarize the current role of GBCA in MRI RADS. This overview suggests that GBCA are today required in most of the current RADS and are expected to be used in most MRIs performed in patients with cancer. Dynamic contrast enhancement is required for correct scores calculation in PI-RADS and VI-RADS, although scientific evidence may lead in the future to avoid the GBCA administration in these two RADS. In Bone-RADS, contrast enhancement can be required to classify an aggressive lesion. In RADS scoring on whole body-MRI datasets (MET-RADS-P, MY-RADS and ONCO-RADS), in NS-RADS and in Node-RADS, GBCA administration is optional thanks to the intrinsic high contrast resolution of MRI. Future studies are needed to evaluate the impact of the high T1 relaxivity GBCA on the assignment of RADS scores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been a large-scale dissemination of clinical reporting guidelines in radiology in the form of Reporting and Data Systems (RADS), which have been proposed as standardized systems for imaging reporting to minimize variations and ambiguous terminology, facilitating images interpretation and outcomes monitoring [1]. Many RADS have been developed by the American College of Radiology (ACR), starting in 1993 with the Breast Imaging-Reporting and Data System (BI-RADS), while some RADS were developed by other groups [2, 3]. The RADS are both modality and technique specific. The role of gadolinium-based contrast agents (GBCA) is still a topic of strong debate, as evidenced by the increasing literature and discussions at international conferences, including the ISMRM-ESMRMB hot topic debate in the 2022 joint annual meeting [4], on the role of GBCA and opportunities for reduced dose and non-contrast imaging. In fact, GBCA administration is often required to achieve an early and accurate diagnosis [5,6,7,8]; in addition, the use of contrast agent can improve the diagnostic ability of less experienced readers. On the other hand, performing examinations without using GBCA has a number of benefits such as: reduced contrast agent-related operational issues, including less pre-MRI patient documentation, blood tests, and safety checks (e.g., allergies and renal function assessments); no concerns regarding potential contrast agent side effects (e.g., contrast extravasation, hematoma, nephrogenic systemic fibrosis, gadolinium deposition, and allergic reactions) [9,10,11]; no contrast agent-related infrastructure (e.g., additional staff, inserting and removing cannulas, and preparing contrast injectors) with cost savings in terms of decreased material and infrastructural use; shortened examination times [12, 13]. In addition, reducing the use of GBCA will reduce their unintended impact on aquatic ecosystems and drinking water resources [14, 15].
The scope of the present review is to summarize the current role of GBCA only in clinical reporting guidelines for MRI that have adopted the “RADS” approach, focusing on three specific questions per each RADS: A. what is the scope of the scoring system; B. how are GBCA used in the scoring system; C. what is the impact of GBCA enhancement on the scoring. Table 1 summarizes the main RADS currently in use in MRI and the role of GBCA.
Literature search strategy
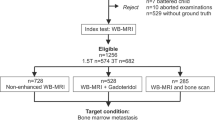
We identified 24 RADS through websites [2, 3]; a subsequent search on PubMed (timeframe between January 1, 2005 and April 29, 2023) was performed, identifying four additional RADS in the literature. Thirteen RADS were excluded, because they did not involve MRI. In the final analysis, we found 15 RADS suitable for use in MRI out of the 28 RADS described in the literature. See Fig. 1 for the flow diagram of the search strategy and study selection and Appendix for details on the search terms used on PubMed. We read the original articles for each latest version of RADS.
Five RADS were endorsed and developed by the ACR: American College of Radiology Breast Imaging-Reporting and Data System (ACR BI-RADS) 5th edition [16, 17], American College of Radiology Liver Imaging-Reporting and Data System (ACR LI-RADS) version 2018 [18], American College of Radiology Neck Imaging-Reporting and Data System (ACR NI-RADS) [19,20,21,22], American College of Radiology Ovarian-Adnexal Reporting and Data System (ACR O-RADS) [23,24,25], and American College of Radiology Prostate Imaging-Reporting and Data System (ACR PI-RADS) version 2.1 [26, 27].
Ten RADS were proposed by other scientific groups: Bone Reporting and Data System (Bone‑RADS) [28], Bone Tumor Imaging-Reporting and Data System (BTI-RADS) [29], Brain Tumor Reporting and Data System (BT-RADS) [30, 31], METastasis Reporting and Data System for Prostate Cancer (MET-RADS-P) [32], Myeloma Response Assessment and Diagnosis System (MY-RADS) [33], Node Reporting and Data System 1.0 (Node-RADS) [34], Neuropathy Score Reporting and Data System (NS-RADS) [35], Oncologically Relevant Findings Reporting and Data System (ONCO-RADS) [36], Osseous Tumor-Reporting and Data System (OT-RADS) [37], and Vesical Imaging-Reporting and Data System (VI-RADS) [38].
Current RADS used in MRI
ACR BI-RADS 5th edition [16, 17, 39, 40]
-
A.
It is a risk assessment and a standardized system of reporting breast pathology that relates categories to management recommendations. It applies to mammography, contrast-enhanced mammography, ultrasound, and contrast-enhanced MRI.
-
B.
T1-weighted dynamic contrast enhancement (DCE) imaging (GBCA dosage of 0.1 mmol/kg body weight) with a maximum acquisition time of 60–120 s per sequence of both breasts is included in the standard MRI protocol combined with bright-fluid and T1-weighted pre-contrast sequences, preferably with fat suppression; subtraction imaging and kinetic curve assessment may be desired. An additional suggested sequence is diffusion weighted imaging (DWI).
-
C.
Enhancement is essential in the assessment of background parenchymal enhancement and in the description of any area of abnormal enhancement, including focus, mass, and non-mass enhancement for the final assignment of the score (from 0 to 6).
ACR LI-RADS v2018 [18, 41,42,43]
-
A.
It is a risk assessment for hepatocellular carcinoma (HCC) and a standardized system of reporting imaging findings in liver lesions of patients with risk factors for HCC, that relates categories to management recommendations. It also allows to assess the response of HCC to locoregional treatment. It applies to contrast-enhanced computed tomography (CT), contrast-enhanced ultrasound, ultrasound, and contrast-enhanced MRI.
-
B.
Multiphase post-contrast T1-weighted imaging (GBCA dosage of 0.1 mmol/kg body weight) [arterial phase (late arterial phase strongly preferred), portal venous phase, delayed phase, and hepatobiliary phase if using gadoxetate disodium (GBCA dosage of 0.025 mmol/kg body weight)] is combined with unenhanced T1-weighted in- and opposed-phase imaging and T2-weighted sequences. Subtraction imaging may be desired. Additional suggested sequences are DWI and 1- to 3-h hepatobiliary phase if using gadobenate dimeglumine (GBCA dosage of 0.1 mmol/kg body weight).
-
C.
Enhancement is essential in the assessment of some major features: arterial phase hyperenhancement, non-peripheral washout, and the presence of an enhancing capsule. Contrast enhancement is also needed for the investigation of some ancillary features: corona enhancement, transitional phase hypointensity, hepatobiliary phase hypointensity or isointensity, mosaic appearance, and parallel blood pool enhancement. In addition, administration of contrast agent allows identification of the targetoid dynamic enhancement (favorable for LR-malignant but not HCC specific) and enhancement of a venous thrombus. Thus, multiphase imaging is critical for the final assignment of the category (from negative to 5 including LR-tumor in vein and LR-malignant but not HCC specific) and to highlight any residual tumor in the post-treatment LI-RADS assignment.
ACR NI-RADS [19,20,21,22]
-
A.
It is a structured head and neck surveillance reporting system after treatment with categories tied to follow up recommendations. Originally developed for surveillance using contrast-enhanced CT with or without PET; NI-RADS can also be applied to contrast-enhanced MRI, mostly for the evaluation of perineural spread.
-
B.
A post-contrast T1-weighted sequence (GBCA dosage of 0.1 mmol/kg body weight) is included in the standard MRI protocol combined with T1-weighted and T2-weighted pre-contrast sequences; an additional suggested sequence is DWI.
-
C.
Enhancement is essential in the evaluation of the primary site and the neck (nodal assessment), in the assignment of all categories (from 1 to 4).
ACR O-RADS [23,24,25, 44, 45]
-
A.
It is a risk assessment and a standardized system of reporting ovarian-adnexal pathology that relates categories to management recommendations. It applies to ultrasound and contrast-enhanced MRI.
-
B.
A DCE-MRI should be performed using a T1-weighted sequence before and after intravenous administration of GBCA (GBCA dosage of 0.1 mmol/kg body weight) to evaluate the time-intensity curves (temporal resolution < 15 s). If DCE-MRI is not possible, then non-DCE-MRI can be performed as a pre- and post-contrast T1-weighted sequence performed 30–40 s after the end of contrast agent injection. Imaging protocol should include at least T2-weighted sequences without fat saturation, T1-weighted in- and opposed-phase images, and DWI.
-
C.
Enhancement is essential in the identification of solid tissue within an adnexal lesion, that raises the suspicion of malignancy. Thus, post-contrast imaging is critical for the final assignment of the category (from 0 to 5) and, in particular, DCE is the key in discriminating between categories 3, 4, and 5 based on the time-intensity curves relative to the outer myometrium.
ACR PI-RADS v2.1 [26, 27, 46, 47]
-
A.
The scope is to improve detection, localization, characterization, and risk stratification in patients with suspected prostate cancer in treatment naïve glands.
-
B.
A DCE with rapid T1-weighted gradient echo sequence (temporal resolution: ≤ 15 s) before, during, and after the intravenous administration of GBCA (GBCA dosage of 0.1 mmol/kg body weight) is currently included in the multi-parameter MRI protocol. Fat suppression or subtraction techniques are proposed to improve the detection of enhancement. Imaging protocol should include at least also a pre-contrast T1-weighted sequence.
-
C.
Although DCE is a component of the multiparametric MRI prostate examination, its role in the determination of PI-RADS v2.1 score is secondary to T2-weighted images and DWI. A positive DCE (defined as focal and earlier than or contemporaneously with enhancement of adjacent normal prostatic tissues and corresponds to suspicious finding on T2-weighted images and/or DWI) upgrades a DWI + PI-RADS 3 in the peripheral zone to PI-RADS 4 (3 + 1). DCE may improve the sensitivity and detection of cancer in both the peripheral and transitional zones, especially when DWI is degraded by artifacts or when less experienced readers are reporting.
Bone‑RADS [28]
-
A.
It is a risk assessment and a standardized system of reporting incidental solitary bone lesions that relates categories to management recommendations. It applies to CT and MRI.
-
B.
Post-contrast imaging is often required to exclude a malignancy, but its evaluation is secondary to pre-contrast T1- and T2-weighted sequences (the latter also with fat suppression).
-
C.
In the presence of a T1 hyperintense solitary bone lesion without macroscopic intralesional fat, the type of contrast enhancement allows to classify the lesion as Bone-RADS 1 (none or thin peripheral enhancement) or Bone-RADS 4 (nodular and/or central enhancement). In the presence of a T1 hypointense solitary bone lesion, the evidence of solid mass enhancement categorizes the lesion as Bone-RADS 4.
BTI-RADS [29]
-
A.
It is a classification system for solitary bone lesions based on various benign and malignant indicators. It applies to CT and MRI.
-
B.
A T1-weighted post-contrast sequence, 5 min after GBCA administration (GBCA dosage of 0.1 mmol/kg body weight), is included in the MRI protocol along with at least two orthogonal T2-weighted with fat suppression images.
-
C.
The absence of contrast enhancement is included in the “benign indicators”, while the type of contrast enhancement (homogenous or heterogeneous) is listed in the “indeterminate features”.
BT-RADS [30, 31, 48]
-
A.
It is a structured primary brain tumor surveillance reporting system with categories tied to management recommendations using contrast-enhanced MRI.
-
B.
A T1-weighted post-contrast sequence (GBCA dosage of 0.1 mmol/kg body weight) is included in the standard MRI protocol combined with T2-weighted Fluid Attenuated Inversion Recovery (FLAIR) sequences; additional suggested sequences are dynamic susceptibility contrast perfusion (to evaluate relative cerebral blood volume) and DWI.
-
C.
Enhancement is essential for the overall assessment of the examination, especially in the assignment of categories 3b, 3c, and 4.
MET-RADS-P [32]
-
A.
The scope is to promote standardization in the reporting of whole body-MRI (WB-MRI) in advanced prostate cancer at the baseline study and in the follow-up of the patient, evaluating response to treatment in metastatic disease. The main purpose is the evaluation of bone and nodal disease, while a more extensive assessment should be used for patients with established visceral disease. The assessment of the prostate or prostatectomy bed is not an essential requirement of the scoring system.
-
B.
Post-contrast imaging is not mandatory in the “core protocol”, when the aim is to obtain information on bone and nodal disease. The main sequences used are: T1-weighted [Turbo Spin Echo (TSE) and Dixon], Short Tau Inversion Recovery (STIR), and DWI.
-
C.
GBCA can be used in a more comprehensive assessment, including dedicated prostate or brain studies.
MY-RADS [33, 49]
-
A.
The scope is to promote standardization in the reporting of WB-MRI in myeloma at the baseline study and in the follow-up of the patient, evaluating response to treatment. The main purpose is the evaluation of bone marrow, while a more extensive assessment should be used for appraisal of soft tissue, extramedullary disease, or for those patients in whom serial tumor response assessments (including clinical trials) are planned.
-
B.
Post-contrast imaging is not mandatory in the “core clinical protocol”, when the aim is to obtain information on bone marrow involvement. The main sequences used are: T1-weighted (TSE and Dixon), STIR, and DWI.
-
C.
GBCA can be used in a more comprehensive assessment, including soft tissue or extramedullary disease evaluation.
Node-RADS [34]
-
A.
The scope is to stratify the risk of having cancer involvement in regional and distant lymph nodes, increasing consensus among radiologists for primary staging and in response assessment settings. It applies to CT and MRI.
-
B.
Post-contrast imaging is not mandatory for MRI because of the intrinsic high soft-tissue contrast, while the use of contrast agents is required for CT scans. The sequences to be evaluated are those where the assessment of the criteria “size” and “configuration” succeeds best.
-
C.
Although it is not strictly necessary for the nodal evaluation, GBCA are often essential in tumor staging and follow-up.
NS-RADS [35, 50]
-
A.
The scope is to improve the reporting and evaluation of peripheral neuropathy on MRI.
-
B.
Post-contrast imaging is not mandatory. The main sequences used are fat-suppressed T2-weighted sequences and either proton density weighted or T1-weighted sequences.
-
C.
Enhancement can be useful especially when there is suspicion of nerve neoplasia (subclass N of the scoring system).
ONCO-RADS [36]
-
A.
The scope is to stratify the risk of having malignant tumors in individuals undergoing WB-MRI for cancer screening in the general population and in predisposition syndromes.
-
B.
Post-contrast imaging is not mandatory for WB-MRI and should be avoided in general population cancer screening. The standard protocol is based on the following sequences: T1-weighted (TSE, Dixon and gradient echo for the lung), STIR, T2-weighted, DWI, and T2-FLAIR (for the brain).
-
C.
GBCA should be used in a more comprehensive assessment when there is a requirement for investigating additional body parts (e.g., soft-tissue mass or breast evaluations) or for brain evaluation in patients with Li-Fraumeni syndrome, neurofibromatosis, constitutional mismatch repair deficiency syndrome, and hereditary retinoblastoma.
OT-RADS [37]
-
A.
The scope is to standardize the classification of osseous tumors to facilitate the differentiation between benign and malignant lesions, achieving good-to-excellent interreader agreement. It applies to MRI.
-
B.
A post-contrast T1-weighted fat-suppressed sequence (GBCA dosage of 0.1 mmol/kg body weight) is required in a “complete MRI study”. Imaging protocol also includes unenhanced T1-weighted images, fat-suppressed T2-weighted or STIR sequences, and DWI.
-
C.
Contrast enhancement features are needed for the final assignment of the score (from II to V). An incomplete examination, for example without GBCA administration, is classified as OT-RADS 0 and requires further imaging.
VI-RADS [38, 51,52,53]
-
A.
It is a systematic approach to reporting MRI of bladder cancer defining, in particular, the risk of muscle layer invasion.
-
B.
A T1-weighted DCE imaging (GBCA dosage of 0.1 mmol/kg body weight) is required before and at 30 s after the beginning of injection and is followed by the same sequences four-to-six times every 30 s to depict the early enhancement of the inner layer followed by tumor enhancement. The late phase is useless in local staging, because signal contrast among the inner and outer layers and tumor decreases. Imaging protocol must include at least T2-weighted sequences without fat saturation and DWI. Quantitative measurements, such as apparent diffusion coefficient measurement and perfusion curves, are optional.
-
C.
DCE is a key component for the final assignment of VI-RADS score (from 1 to 5). The final category is first based on T2-weighted sequences for the morphology. The presence of definitive muscular invasion is decided using DWI and DCE-MRI (especially when DWI is tainted by artifacts).
Discussion
This overview suggests that GBCA are today required in most of the current RADS and, thus, are expected to be used in most MRIs performed in patients with cancer. Several authors, different from those who have proposed RADS, have analyzed the possible role of unenhanced MRI (uMRI) in RADS protocols also with the support of artificial intelligence, aiming to carry gadolinium-analog information. As an example, regarding BI-RADS, unenhanced MRI with STIR and DWI sequences had the same diagnostic performance compared to contrast-enhanced MRI in patients with BI-RADS 0 (lesions that need further investigation for complete analysis) [54]. Another study, comparing uMRI + Digital Breast Tomosynthesis (DBT) with DCE-MRI, concluded that DCE-MRI was the most sensitive imaging technique in breast cancer preoperative assessment despite the good accuracy of uMRI + DBT [55]. In a 3.0 T scanner setting, diagnostic performance and interreader agreement of both uMRI and DCE-MRI were high, with inferior lesion conspicuity and lower interreader agreement of uMRI [56].
Regarding the O-RADS, a recent study has highlighted that the morphological and qualitative DWI assessment by gynecological radiologists could be an alternative when intravenous contrast agent and a dynamic curve assessment for the formal O–RADS score cannot be provided [57].
In recent years, many studies have investigated the possible role of uMRI in PI-RADS, based on T2-weighted and DWI sequences (so-called biparametric MRI) in patients with treatment naïve prostate cancer. Most works compared uMRI versus DCE-MRI in screening and evaluating prostatic gland, showing that contrast enhancement had no or only a marginal effect on the diagnostic performance for detecting clinically significant cancers. However, the role of DCE-MRI in increasing the score from PI-RADS 3 to PI-RADS 4, the diagnostic impact of GBCA enhancement when T2-weighted and DWI sequences are degraded by artifacts, or the importance of contrast agents in helping radiologists with less experience were not often evaluated [58]. A simplified PI-RADS based on biparametric MRI has also been proposed, with the scope to assist radiologists and urologists in the detection and management of prostatic cancer [59]. Additionally, artificial intelligence tools have been exponentially developed in the setting of prostate cancer diagnosis by taking advantage of the opportunities of uMRI. A recent review summarized the role of machine-learning or deep-learning in biparametric prostate MRI, showing promising results in terms of cancer detection and differentiation from non-cancerous tissue. However, the authors pointed out that there was still great variability between reports and only a few multicenter studies were available [60,61,62]. Therefore, if machine-learning could help in avoiding the systematic use of GBCA in prostate MRI and the use of deep learning-based softwares could improve reporting times, the clinical applicability of these approaches still requires more robust validation across scanner vendors, field strengths, and institutions. The PI-RADS Committee concluded that the biparametric MRI requires optimal image acquisition and data interpretation, the possibility of instituting patient recalls or on-table monitoring of images when there is insufficient quality and in indeterminate cases. As an alternative approach, it remains desirable to tailor the need for GBCA-enhanced MRI according to patient risk. In fact, men at intermediate risk might undergo contrast-enhanced MRI as the default approach. The PI-RADS Committee underlined the need for further higher quality data before issuing evidence-based recommendations about unenhanced MRI as an initial diagnostic approach for prostate cancer workup [13].
Similar to PI-RADS, an alternative “biparametric” protocol has been proposed in VI-RADS, consisting only of T2-weighted and DWI sequences without the use of GBCA, employing 1.5 and 3.0 T MRI scanners, with a comparable diagnostic accuracy to the standard MRI protocol for the detection of muscle-invasive bladder cancer [63,64,65,66]; a meta-analysis confirmed these results [67]. In these cases, a denoising deep-learning reconstruction could significantly improve the diagnostic accuracy of T2-weighted sequences [68]. On the other hand, the use of DCE-MRI could provide additional value to the radiomics-based identification of muscle-invasive cancer [69].
Although these studies show promising results, the use of artificial intelligence applied to the field of RADS in clinical practice is still uncommon.
Another aspect to consider relates to the increasing use of high T1 relaxivity contrast agents, which allow reducing the GBCA dose without compromising image quality and diagnostic accuracy. Gadopiclenol 0.5 mmol/ml is a new GBCA approved for clinical use in September 2022 by the Food and Drug Administration at a dosage of 0.05 mmol/kg body weight [70]. It is based on a pyclen macrocyclic structure, offering good stabilities (thermodynamic and kinetic) and high r1 relaxivity (about twofold that of other macrocyclic GBCA). These characteristics confer a double benefit: use half of the standard dose to get the same efficacy and use the standard dose to get a higher enhancement [71]. Another high T1 relaxivity contrast agent, named gadoquatrane, is currently under development with a preclinical profile characterized by favorable physicochemical properties and the same pharmacokinetic profile as currently used GBCA; thus, gadoquatrane represents an excellent candidate for further clinical development [72]. In most oncology imaging, and therefore also in the RADS for MRI that we have listed, 0.1 mmol/kg body weight of GBCA is used, delivered at a flow rate of 2–4 mL/s [73]. In particular, the signal change observed in DCE-MRI depends on the concentration of the contrast agent applied; at low-to-modest GBCA concentrations T1 shortening leads to signal increases, while at high GBCA concentrations, signal losses due to T2* shortening occur, affecting, for example, the time–intensity curves [74]. Therefore, the use of these new high-relaxivity GBCA could affect the longitudinal evaluation of oncological patients when examinations performed with different classes of contrast agent are compared. Future studies are needed to evaluate the impact of these new GBCA on the assignment of RADS scores, especially when DCE is required.
Conclusion
-
Currently GBCA administration plays a key role in most MRI RADS.
-
Dynamic contrast enhancement is required for score calculation in ACR PI-RADS and VI-RADS, although scientific evidence may lead in the future to avoid the GBCA administration in these two RADS also with the aid of artificial intelligence tools.
-
In Bone-RADS contrast enhancement is often required to classify a lesion as Bone-RADS 4.
-
In RADS where WB-MRI is applied (MET-RADS-P, MY-RADS, and ONCO-RADS), in NS-RADS and in Node-RADS, GBCA is optional due to the intrinsic high contrast resolution of MRI.
-
Future studies are needed to evaluate the impact of the next-generation high T1 relaxivity GBCA on the assignment of RADS scores.
Data availability
No new data are associated with this review article.
Abbreviations
- ACR:
-
American College of Radiology
- BI-RADS:
-
Breast imaging reporting and data system
- Bone-RADS:
-
Bone reporting and data system
- BTI-RADS:
-
Bone tumor imaging reporting and data system
- BT-RADS:
-
Brain tumor reporting and data system
- CT:
-
Computed tomography
- DBT:
-
Digital breast tomosynthesis
- DCE:
-
Dynamic contrast enhancement
- DWI:
-
Diffusion weighted imaging
- ESMRMB:
-
European Society for Magnetic Resonance in Medicine and Biology
- FLAIR:
-
Fluid attenuated inversion recovery
- GBCA:
-
Gadolinium-based contrast agent
- GREC:
-
Gadolinium Research and Education Committee
- HCC:
-
Hepatocellular carcinoma
- ISMRM:
-
International Society for Magnetic Resonance in Medicine
- LI-RADS:
-
Liver imaging reporting and data system
- MET-RADS-P:
-
METastasis reporting and data system for prostate cancer
- MRI:
-
Magnetic resonance imaging
- MY-RADS:
-
Myeloma response assessment and diagnosis system
- NI-RADS:
-
Neck imaging reporting and data system
- Node-RADS:
-
Node Reporting and Data System 1.0
- NS-RADS:
-
Neuropathy score reporting and data system
- ONCO-RADS:
-
Oncologically relevant findings reporting and data system
- O-RADS:
-
Ovarian-adnexal reporting and data system
- OT-RADS:
-
Osseous tumor reporting and data system
- PI-RADS:
-
Prostate imaging reporting and data system
- RADS:
-
Reporting and data systems
- STIR:
-
Short tau inversion recovery
- TSE:
-
Turbo spine echo
- uMRI:
-
Unenhanced MRI
- VI-RADS:
-
Vesical imaging reporting and data system
- WB-MRI:
-
Whole body-magnetic resonance imaging
References
An JY, Unsdorfer KML, Weinreb JC (2019) BI-RADS, C-RADS, CAD-RADS, LI-RADS, Lung-RADS, NI-RADS, O-RADS, PI-RADS, TI-RADS: reporting and data systems. Radiographics 39:1435–1436
Bell DJ (2005) Reporting and data systems (disambiguation). In: Radiology reference article. Radiopaedia.org. Radiopaedia. https://doi.org/10.53347/rID-76158
Reporting and Data Systems. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems. Accessed 14 Dec 2022
Quattrocchi CC, Agarwal N, Taso M, Dekkers IA (2022) Report on the ISMRM-ESMRMB 2022 hot topic debate on the future of gadolinium as a contrast agent. MAGMA 35:707–710
Parillo M, Vaccarino F, Quattrocchi CC (2023) Imaging findings in a case of leptomeningeal myelomatosis, a rare but critical central nervous system complication of multiple myeloma. Neuroradiol J. https://doi.org/10.1177/19714009221150849
Parillo M, Bernetti C, Altomare C, Beomonte Zobel B, Quattrocchi CC (2023) Extrahepatic abscess and dropped gallstones: a case report and a narrative review of an unusual delayed complication of laparoscopic cholecystectomy. Acta Chir Belg. https://doi.org/10.1080/00015458.2022.2163957
De Stefano D, Parillo M, Garipoli A, Beomonte Zobel B (2021) Imaging findings in a case of myo-pericarditis associated with SARS CoV-2 disease. J Cardiol Cases 24:210–214
Parillo M, Vertulli D, Mallio CA, Quattrocchi CC (2023) Imaging findings in carcinomatous encephalitis secondary to malignant melanoma. Egypt J Neurol Psychiatry Neurosurg 59:76
Parillo M, Mallio CA, Van der Molen AJ, Rovira À, Ramalho J, Ramalho M, Gianolio E, Karst U, Radbruch A, Stroomberg G, Clement O, Dekkers IA, Nederveen AJ, Quattrocchi CC, ESMRMB-GREC Working Group (2023) Skin toxicity after exposure to gadolinium-based contrast agents in normal renal function, using clinical approved doses: current status of preclinical and clinical studies. Invest Radiol. https://doi.org/10.1097/RLI.0000000000000973
Parillo M, Sapienza M, Arpaia F, Magnani F, Mallio CA, DʼAlessio P, Quattrocchi CC (2019) A structured survey on adverse events occurring within 24 hours after intravenous exposure to gadodiamide or gadoterate meglumine: a controlled prospective comparison study. Invest Radiol 54:191–197
Quattrocchi CC, Parillo M, Spani F, Landi D, Cola G, Dianzani C, Perrella E, Marfia GA, Mallio CA (2023) Skin thickening of the scalp and high signal intensity of dentate nucleus in multiple sclerosis: association with linear versus macrocyclic gadolinium-based contrast agents administration. Invest Radiol 58:223–230
ESUR Guidelines 10 (2018). https://www.esur.org/wp-content/uploads/2022/03/ESUR-Guidelines-10_0-Final-Version.pdf. Accessed 10 May 2023
Schoots IG, Barentsz JO, Bittencourt LK, Haider MA, Macura KJ, Margolis DJA, Moore CM, Oto A, Panebianco V, Siddiqui MM, Tempany C, Turkbey B, Villeirs GM, Weinreb JC, Padhani AR (2021) PI-RADS committee position on MRI without contrast medium in biopsy-naive men with suspected prostate cancer: narrative review. AJR Am J Roentgenol 216:3–19
Birka M, Wehe CA, Hachmöller O, Sperling M, Karst U (2016) Tracing gadolinium-based contrast agents from surface water to drinking water by means of speciation analysis. J Chromatogr A 1440:105–111
Schmidt K, Bau M, Merschel G, Tepe N (2019) Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci Total Environ 687:1401–1408
American College of Radiology Committee on BI-RADS® (2013). https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/MRI-Reporting.pdf
Morris EA, Comstock CE, Lee CH et al (2013) ACR BI-RADS® magnetic resonance imaging ACR BI-RADS® atlas, breast imaging reporting and data system. American College of Radiology, Reston, VA
American College of Radiology Committee on LI-RADS® (2018). https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf. Accessed 10 May 2023
Aiken AH, Hudgins PA (2018) Neck imaging reporting and data system. Magn Reson Imaging Clin N Am 26:51–62
Aiken AH, Farley A, Baugnon KL, Corey A, El-Deiry M, Duszak R, Beitler J, Hudgins PA (2016) Implementation of a novel surveillance template for head and neck cancer: neck imaging reporting and data system (NI-RADS). J Am Coll Radiol 13:743-746.e1
Aiken AH, Rath TJ, Anzai Y, Branstetter BF, Hoang JK, Wiggins RH, Juliano AF, Glastonbury C, Phillips CD, Brown R, Hudgins PA (2018) ACR neck imaging reporting and data systems (NI-RADS): a white paper of the ACR NI-RADS committee. J Am Coll Radiol 15:1097–1108
American College of Radiology Committee on NI-RADSTM (2021). https://www.acr.org/-/media/ACR/Files/RADS/NI-RADS/ACR-NI-RADS-MRI-Table.pdf. Accessed 10 May 2023
Sadowski EA, Thomassin-Naggara I, Rockall A, Maturen KE, Forstner R, Jha P, Nougaret S, Siegelman ES, Reinhold C (2022) O-RADS MRI risk stratification system: guide for assessing adnexal lesions from the ACR O-RADS committee. Radiology 303:35–47
Reinhold C, Rockall A, Sadowski EA, Siegelman ES, Maturen KE, Vargas HA, Forstner R, Glanc P, Andreotti RF, Thomassin-Naggara I (2021) Ovarian-adnexal reporting lexicon for MRI: a white paper of the ACR ovarian-adnexal reporting and data systems MRI committee. J Am Coll Radiol 18:713–729
American College of Radiology Committee on O-RADSTM (2021). https://www.acr.org/-/media/ACR/Files/RADS/O-RADS/O-RADS-MR-Lexicon-Terms-Table.pdf. Accessed 10 May 2023
Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, Tempany CM, Choyke PL, Cornud F, Margolis DJ, Thoeny HC, Verma S, Barentsz J, Weinreb JC (2019) Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 76:340–351
American College of Radiology Committee on PI-RADSTM (2019). https://www.acr.org/-/media/ACR/Files/RADS/PI-RADS/PIRADS-V2-1.pdf. Accessed 10 May 2023
Chang CY, Garner HW, Ahlawat S, Amini B, Bucknor MD, Flug JA, Khodarahmi I, Mulligan ME, Peterson JJ, Riley GM, Samim M, Lozano-Calderon SA, Wu JS (2022) Society of Skeletal Radiology-white paper. Guidelines for the diagnostic management of incidental solitary bone lesions on CT and MRI in adults: bone reporting and data system (Bone-RADS). Skeletal Radiol. https://doi.org/10.1007/s00256-022-04022-8
Ribeiro GJ, Gillet R, Hossu G, Trinh J-M, Euxibie E, Sirveaux F, Blum A, Teixeira PAG (2021) Solitary bone tumor imaging reporting and data system (BTI-RADS): initial assessment of a systematic imaging evaluation and comprehensive reporting method. Eur Radiol 31:7637–7652
Weinberg BD, Gore A, Shu H-KG, Olson JJ, Duszak R, Voloschin AD, Hoch MJ (2018) Management-based structured reporting of posttreatment glioma response with the brain tumor reporting and data system. J Am Coll Radiol 15:767–771
Home-Brain Tumor Reporting and Data System (BT-RADS). In: Brain tumor rads (BT-Rads). https://btrads.com/. Accessed 16 Oct 2022
Padhani AR, Lecouvet FE, Tunariu N, Koh D-M, De Keyzer F, Collins DJ, Sala E, Schlemmer HP, Petralia G, Vargas HA, Fanti S, Tombal HB, de Bono J (2017) METastasis reporting and data system for prostate cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. Eur Urol 71:81–92
Messiou C, Hillengass J, Delorme S, Lecouvet FE, Moulopoulos LA, Collins DJ, Blackledge MD, Abildgaard N, Østergaard B, Schlemmer H-P, Landgren O, Asmussen JT, Kaiser MF, Padhani A (2019) Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS). Radiology 291:5–13
Elsholtz FHJ, Asbach P, Haas M, Becker M, Beets-Tan RGH, Thoeny HC, Padhani AR, Hamm B (2021) Introducing the node reporting and data system 1.0 (Node-RADS): a concept for standardized assessment of lymph nodes in cancer. Eur Radiol 31:6116–6124
Chhabra A, Deshmukh SD, Lutz AM, Fritz J, Andreisek G, Sneag DB, Subhawong T, Singer AD, Wong PK, Thakur U, Pandey T, Chalian M, Mogharrabi BN, Guirguis M, Xi Y, Ahlawat S (2022) Neuropathy score reporting and data system: a reporting guideline for MRI of peripheral neuropathy with a multicenter validation study. AJR Am J Roentgenol 219:279–291
Petralia G, Koh D-M, Attariwala R, Busch JJ, Eeles R, Karow D, Lo GG, Messiou C, Sala E, Vargas HA, Zugni F, Padhani AR (2021) Oncologically relevant findings reporting and data system (ONCO-RADS): guidelines for the acquisition, interpretation, and reporting of whole-body MRI for cancer screening. Radiology 299:494–507
Chhabra A, Gupta A, Thakur U, Pezeshk P, Dettori N, Callan A, Xi Y, Weatherall P (2021) Osseous tumor reporting and data system-multireader validation study. J Comput Assist Tomogr 45:571–585
Panebianco V, Narumi Y, Altun E, Bochner BH, Efstathiou JA, Hafeez S, Huddart R, Kennish S, Lerner S, Montironi R, Muglia VF, Salomon G, Thomas S, Vargas HA, Witjes JA, Takeuchi M, Barentsz J, Catto JWF (2018) Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (vesical imaging-reporting and data system). Eur Urol 74:294–306
Spick C, Bickel H, Polanec SH, Baltzer PA (2018) Breast lesions classified as probably benign (BI-RADS 3) on magnetic resonance imaging: a systematic review and meta-analysis. Eur Radiol 28:1919–1928
Li J, Zheng H, Cai W, Wang Y, Zhang H, Liao M (2020) Subclassification of BI-RADS 4 magnetic resonance lesions: a systematic review and meta-analysis. J Comput Assist Tomogr 44:914–920
Shin J, Lee S, Yoon JK, Son WJ, Roh YH, Chung YE, Choi J-Y, Park M-S (2022) Diagnostic performance of LI-RADS v2018 versus KLCA-NCC 2018 criteria for hepatocellular carcinoma using magnetic resonance imaging with hepatobiliary agent: a systematic review and meta-analysis of comparative studies. Gut Liver. https://doi.org/10.5009/gnl220115
Kim Y-Y, Lee S, Shin J, Son WJ, Roh YH, Hwang JA, Lee JE (2022) Diagnostic performance of CT versus MRI liver imaging reporting and data system category 5 for hepatocellular carcinoma: a systematic review and meta-analysis of comparative studies. Eur Radiol 32:6723–6729
van der Pol CB, McInnes MDF, Salameh J-P, Chernyak V, Tang A, Bashir MR, LI-RADS IPD Group, LI-RADS IPD Group Collaborators (2022) Impact of reference standard on CT, MRI, and contrast-enhanced US LI-RADS diagnosis of hepatocellular carcinoma: a meta-analysis. Radiology 303:544–545
Rizzo S, Cozzi A, Dolciami M, Del Grande F, Scarano AL, Papadia A, Gui B, Gandolfo N, Catalano C, Manganaro L (2022) O-RADS MRI: a systematic review and meta-analysis of diagnostic performance and category-wise malignancy rates. Radiology 307:e220795
Vara J, Manzour N, Chacón E, López-Picazo A, Linares M, Pascual MÁ, Guerriero S, Alcázar JL (2022) Ovarian adnexal reporting data system (O-RADS) for classifying adnexal masses: a systematic review and meta-analysis. Cancers (Basel) 14:3151
Wen J, Ji Y, Han J, Shen X, Qiu Y (2022) Inter-reader agreement of the prostate imaging reporting and data system version v2.1 for detection of prostate cancer: a systematic review and meta-analysis. Front Oncol 12:1013941
Oerther B, Engel H, Bamberg F, Sigle A, Gratzke C, Benndorf M (2022) Cancer detection rates of the PI-RADSv2.1 assessment categories: systematic review and meta-analysis on lesion level and patient level. Prostate Cancer Prostatic Dis 25:256–263
Parillo M, Mallio CA, Pileri M, Dirawe D, Romano A, Bozzao A, Weinberg B, Quattrocchi CC (2023) Interrater reliability of brain tumor reporting and data system (BT-RADS) in the follow up of adult primary brain tumors: a single institution experience in Italy. Quant Imaging Med Surg. https://doi.org/10.21037/qims-22-850
Rata M, Blackledge M, Scurr E, Winfield J, Koh D-M, Dragan A, Candito A, King A, Rennie W, Gaba S, Suresh P, Malcolm P, Davis A, Nilak A, Shah A, Gandhi S, Albrizio M, Drury A, Roberts S, Jenner M, Brown S, Kaiser M, Messiou C (2022) Implementation of whole-body MRI (MY-RADS) within the OPTIMUM/MUKnine multi-centre clinical trial for patients with myeloma. Insights Imaging 13:123
Chhabra A, Deshmukh SD, Lutz AM, Fritz J, Sneag DB, Mogharrabi B, Guirguis M, Andreisek G, Xi Y, Ahlawat S (2022) Neuropathy score reporting and data system (NS-RADS): MRI reporting guideline of peripheral neuropathy explained and reviewed. Skeletal Radiol 51:1909–1922
Jazayeri SB, Dehghanbanadaki H, Hosseini M, Taghipour P, Bazargani S, Thomas D, Feibus A, Sarabchian E, Bacchus MW, Di Valerio EA, Bandyk M, Balaji KC (2022) Inter-reader reliability of the vesical imaging-reporting and data system (VI-RADS) for muscle-invasive bladder cancer: a systematic review and meta-analysis. Abdom Radiol (NY) 47:4173–4185
Del Giudice F, Flammia RS, Pecoraro M, Moschini M, D’Andrea D, Messina E, Pisciotti LM, De Berardinis E, Sciarra A, Panebianco V (2022) The accuracy of vesical imaging-reporting and data system (VI-RADS): an updated comprehensive multi-institutional, multi-readers systematic review and meta-analysis from diagnostic evidence into future clinical recommendations. World J Urol 40:1617–1628
Woo S, Panebianco V, Narumi Y, Del Giudice F, Muglia VF, Takeuchi M, Ghafoor S, Bochner BH, Goh AC, Hricak H, Catto JWF, Vargas HA (2020) Diagnostic performance of vesical imaging reporting and data system for the prediction of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur Urol Oncol 3:306–315
Zhang R, Xu M, Zhou C, Ding X, Lu H, Ge M, Du L, Bu Y (2022) The value of noncontrast MRI in evaluating breast imaging reporting and data system category 0 lesions on digital mammograms. Quant Imaging Med Surg 12:4069–4080
Rizzo V, Moffa G, Kripa E, Caramanico C, Pediconi F, Galati F (2021) Preoperative staging in breast cancer: intraindividual comparison of unenhanced MRI combined with digital breast tomosynthesis and dynamic contrast enhanced-MRI. Front Oncol 11:661945
Baltzer PAT, Bickel H, Spick C, Wengert G, Woitek R, Kapetas P, Clauser P, Helbich TH, Pinker K (2018) Potential of noncontrast magnetic resonance imaging with diffusion-weighted imaging in characterization of breast lesions: intraindividual comparison with dynamic contrast-enhanced magnetic resonance imaging. Invest Radiol 53:229–235
Sahin H, Panico C, Ursprung S, Simeon V, Chiodini P, Frary A, Carmo B, Smith J, Freeman S, Jimenez-Linan M, Bolton H, Haldar K, Ang JE, Reinhold C, Sala E, Addley H (2021) Non-contrast MRI can accurately characterize adnexal masses: a retrospective study. Eur Radiol 31:6962–6973
Belue MJ, Yilmaz EC, Daryanani A, Turkbey B (2022) Current status of biparametric MRI in prostate cancer diagnosis: literature analysis. Life (Basel) 12:804
Scialpi M, Scialpi P, Martorana E, Torre R, Improta A, Aisa MC, D’Andrea A, Di Blasi A (2021) Simplified PI-RADS (S-PI-RADS) for biparametric MRI to detect and manage prostate cancer: What urologists need to know. Turk J Urol 47:175–182
Michaely HJ, Aringhieri G, Cioni D, Neri E (2022) Current value of biparametric prostate MRI with machine-learning or deep-learning in the detection, grading, and characterization of prostate cancer: a systematic review. Diagnostics (Basel) 12:799
Cipollari S, Pecoraro M, Forookhi A, Laschena L, Bicchetti M, Messina E, Lucciola S, Catalano C, Panebianco V (2022) Biparametric prostate MRI: impact of a deep learning-based software and of quantitative ADC values on the inter-reader agreement of experienced and inexperienced readers. Radiol Med 127:1245–1253
Cuocolo R, Cipullo MB, Stanzione A, Ugga L, Romeo V, Radice L, Brunetti A, Imbriaco M (2019) Machine learning applications in prostate cancer magnetic resonance imaging. Eur Radiol Exp 3:35
Watanabe M, Taguchi S, Machida H, Tambo M, Takeshita Y, Kariyasu T, Fukushima K, Shimizu Y, Okegawa T, Fukuhara H, Yokoyama K (2022) Clinical validity of non-contrast-enhanced VI-RADS: prospective study using 3-T MRI with high-gradient magnetic field. Eur Radiol. https://doi.org/10.1007/s00330-022-08813-4
Aslan S, Cakir IM, Oguz U, Bekci T, Demirelli E (2022) Comparison of the diagnostic accuracy and validity of biparametric MRI and multiparametric MRI-based VI-RADS scoring in bladder cancer; is contrast material really necessary in detecting muscle invasion? Abdom Radiol (NY) 47:771–780
Delli Pizzi A, Mastrodicasa D, Marchioni M, Primiceri G, Di Fabio F, Cianci R, Seccia B, Sessa B, Mincuzzi E, Romanelli M, Castellan P, Castellucci R, Colasante A, Schips L, Basilico R, Caulo M (2021) Bladder cancer: do we need contrast injection for MRI assessment of muscle invasion? A prospective multi-reader VI-RADS approach. Eur Radiol 31:3874–3883
Elshetry ASF, El-Fawakry RM, Hamed EM, Metwally MI, Zaid NA (2022) Diagnostic accuracy and discriminative power of biparametric versus multiparametric MRI in predicting muscle-invasive bladder cancer. Eur J Radiol 151:110282
Ye L, Chen Y, Xu H, Xie H, Yao J, Liu J, Song B (2022) Biparametric magnetic resonance imaging assessment for detection of muscle-invasive bladder cancer: a systematic review and meta-analysis. Eur Radiol 32:6480–6492
Taguchi S, Tambo M, Watanabe M, Machida H, Kariyasu T, Fukushima K, Shimizu Y, Okegawa T, Yokoyama K, Fukuhara H (2021) Prospective validation of vesical imaging-reporting and data system using a next-generation magnetic resonance imaging scanner-is denoising deep learning reconstruction useful? J Urol 205:686–692
Liu Y, Xu X, Wang H, Liu Y, Wang Y, Dong Q, Li Z, Guo Y, Lu H (2022) The additional value evaluation of tri-parametric MRI in identifying muscle-invasive status in bladder cancer. Acad Radiol S1076–6332(22):00261–00266
FDA Gadopiclenol (2022). https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216986s000lbl.pdf. Accessed 10 May 2023
Lancelot E, Raynaud J-S, Desché P (2020) Current and future MR contrast agents: seeking a better chemical stability and relaxivity for optimal safety and efficacy. Invest Radiol 55:578–588
Lohrke J, Berger M, Frenzel T, Hilger C-S, Jost G, Panknin O, Bauser M, Ebert W, Pietsch H (2022) Preclinical profile of gadoquatrane: a novel tetrameric, macrocyclic high relaxivity gadolinium-based contrast agent. Invest Radiol 57:629–638
Shukla-Dave A, Obuchowski NA, Chenevert TL, Jambawalikar S, Schwartz LH, Malyarenko D, Huang W, Noworolski SM, Young RJ, Shiroishi MS, Kim H, Coolens C, Laue H, Chung C, Rosen M, Boss M, Jackson EF (2019) Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J Magn Reson Imaging 49:e101–e121
Petralia G, Summers PE, Agostini A, Ambrosini R, Cianci R, Cristel G, Calistri L, Colagrande S (2020) Dynamic contrast-enhanced MRI in oncology: how we do it. Radiol Med 125:1288–1300
Acknowledgements
The ESMRMB-GREC (Gadolinium Research and Education Committee) is a group of multidisciplinary ESMRMB members, including clinicians, scientists, chemists, physicists, pathologists, and clinical epidemiologists who all share a common interest in the study of gadolinium-based contrast agents in a wide variety of clinical and preclinical conditions. The authors would like to thank all the members for their insights despite their specific competence and experience. The list of authors includes the members who developed the project and those experts who have revised and approved the final version of the manuscript.
Funding
Open access funding provided by Università degli Studi di Trento within the CRUI-CARE Agreement. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by MP and CCQ. The first draft of the manuscript was written by MP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The ESMRMB-GREC (Gadolinium Research and Education Committee) is a group of multidisciplinary ESMRMB members, including academic experts of industries. GREC annual meetings are sponsored by the unconditional support of Bayer AG, Bracco, GE Healthcare, and Guerbet.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Parillo, M., Mallio, C.A., Van der Molen, A.J. et al. The role of gadolinium-based contrast agents in magnetic resonance imaging structured reporting and data systems (RADS). Magn Reson Mater Phy 37, 15–25 (2024). https://doi.org/10.1007/s10334-023-01113-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10334-023-01113-y