Abstract
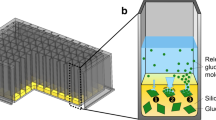
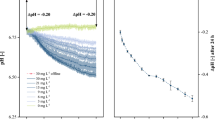
Batch and fed-batch operation result in completely different physiological conditions for cultivated microorganisms or cells. To close the gap between screening, which is hitherto exclusively performed in batch mode, and fed-batch production processes, a special microtiter plate was developed that allows screening in fed-batch mode. The fed-batch microtiter plate (FB-MTP) enables 44 parallel fed-batch experiments at small scale. A small channel filled with a hydrogel connects a reservoir well with a culture well. The nutrient compound diffuses from the reservoir well through the hydrogel into the culture well. Hence, the feed rate can easily be adjusted to the needs of the cultured microorganisms by changing the geometry of the hydrogel channel and the driving concentration gradient. Any desired compound including liquid nutrients like glycerol can be fed to the culture. In combination with an optical measuring device (BioLector), online monitoring of these 44 fed-batch cultures is possible. Two Escherichia coli strains and a Hansenula polymorpha strain were successfully cultivated in the new FB-MTP. As a positive impact of the fed-batch mode on the used strains, a fourfold increase in product formation was observed for E. coli. For H. polymorpha, the use of fed-batch mode resulted in a strong increase in product formation, whereas no measurable product formation was observed in batch mode. In conclusion, the newly developed fed-batch microtiter plate is a versatile, easy-to-use, disposable system to perform fed-batch cultivations at small scale. Screening cultures in high-throughput under online monitoring are possible similar to cultivations under production conditions.







Similar content being viewed by others
References
Amuel C, Gellissen G, Hollenberg CP, Suckow M (2000) Analysis of heat shock promoters in Hansenula polymorpha: the TPS1 promoter, a novel element for heterologous gene expression. Biotechnol Bioprocess Eng 5(4):247–252
Bähr C, Leuchtle B, Lehmann C, Becker J, Jeude M, Peinemann F, Arbter R, Büchs J (2012) Dialysis shake flask for effective screening in fed-batch mode. Biochem Eng J 69:182–195
Bertani G (1951) Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62(3):293–300
Bertani G (2004) Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J Bacteriol 186(3):595–600
Broughall JM, Anslow PA, Kilsby DC (1983) Hazard analysis applied to microbial-growth in food: development of mathematical-models describing the effect of water activity. J Appl Bacteriol 55(1):101–110
Büchs J (2001) Introduction to advantages and problems of shaken cultures. Biochem Eng J 7(2):91–98
De Deken RH (1966) Crabtree effect: a regulatory system in yeast. J Gen Microbiol 44(2):149–156
Drepper T, Eggert T, Circolone F, Heck A, Krauß U, Guterl J-K, Wendorff M, Losi A, Gärtner W, Jaeger K-E (2007) Reporter proteins for in vivo fluorescence without oxygen. Nat Biotechnol 25(4):443–445
Drepper T, Huber R, Heck A, Circolone F, Hillmer A-K, Büchs J, Jaeger K-E (2010) Flavin mononucleotide-based fluorescent reporter proteins outperform green fluorescent protein-like proteins as quantitative in vivo real-time reporters. Appl Environ Microbiol 76(17):5990–5994
Fick A (1855) Ueber diffusion. Ann Phys 170(1):59–86
Funke M, Buchenauer A, Mokwa W, Kluge S, Hein L, Mueller C, Kensy F, Büchs J (2010) Bioprocess control in microscale: scalable fermentations in disposable and user-friendly microfluidic systems. Microb Cell Fact 9:86
Funke M, Buchenauer A, Schnakenberg U, Mokwa W, Diederichs S, Mertens A, Müller C, Kensy F, Büchs J (2010) Microfluidic BioLector––microfluidic bioprocess control in microtiter plates. Biotechnol Bioeng 107(3):497–505
Gellissen G (2000) Heterologous protein production in methylotrophic yeasts. Appl Microbiol Biotechnol 54(6):741–750
Gellissen G (2004) Production of recombinant proteins: novel microbial and eukaryotic expression system. Wiley-VCH, Weinheim
Hermann R, Lehmann M, Büchs J (2003) Characterization of gas-liquid mass transfer phenomena in microtiter plates. Biotechnol Bioeng 81(2):178–186
Huber R, Ritter D, Hering T, Hillmer A-K, Kensy F, Müller C, Wang L, Büchs J (2009) Robo-Lector: a novel platform for automated high-throughput cultivations in microtiter plates with high information content. Microb Cell Fact 8:42
Jacob F, Monod J (1961) Genetic regulatory mechanisms in synthesis of proteins. J Mol Biol 3(3):318–356
Jeude M, Dittrich B, Niederschulte H, Anderlei T, Knocke C, Klee D, Büchs J (2006) Fed-batch mode in shake flasks by slow-release technique. Biotechnol Bioeng 95(3):433–445
Katzke N, Arvani S, Bergmann R, Circolone F, Markert A, Svensson V, Jaeger K-E, Heck A, Drepper T (2010) A novel T7 RNA polymerase dependent expression system for high-level protein production in the phototrophic bacterium Rhodobacter capsulatus. Prot Expr Purif 69(2):137–146
Kedem O, Katchalsky A (1958) Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta 27(2):229–246
Kedem O, Katchalsky A (1963) Permeablility of composite membranes. 1. Electric current, volume flow of solute through membranes. Trans Faraday Soc 59(488):1918–1930
Kensy F, Zang E, Faulhammer C, Tan R-K, Büchs J (2009) Validation of a high-throughput fermentation system based on online monitoring of biomass and fluorescence in continuously shaken microtiter plates. Microb Cell Fact 8:31
Kensy F, Zimmermann HF, Knabben I, Anderlei T, Trauthwein H, Dingerdissen U, Büchs J (2005) Oxygen transfer phenomena in 48-well microtiter plates: determination by optical monitoring of sulfite oxidation and verification by real-time measurement during microbial growth. Biotechnol Bioeng 89(6):698–708
Kim BS, Lee SC, Lee SY, Chang YK, Chang HN (2004) High cell density fed-batch cultivation of E. coli using exponential feeding combined with pH-stat. Bioproc Biosyst Eng 26(3):147–150
Kottmeier K, Weber J, Mueller C, Bley T, Büchs J (2009) Asymmetric division of H. polymorpha reflected by a drop of light scatter intensity measured in batch microtiter plate cultivations at phosphate limitation. Biotechnol Bioeng 104(3):554–561
Krause M, Ukkonen K, Haataja T, Ruottinen M, Glumoff T, Neubauer A, Neubauer P, Vasala A (2010) A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures. Microb Cell Fact 9:11
Kumar S, Wittmann C, Heinzle E (2004) Mini-bioreactors. Biotechnol Lett 26(1):1–10
Larsson G, Jørgensen SB, Pons MN, Sonnleitner B, Tijsterman A, Titchener-Hooker N (1997) Biochemical engineering science. J Biotechnol 59(1–2):3–9
McGown EL, Hafeman DG (1998) Multichannel pipettor performance verified by measuring pathlength of reagent dispensed into a microplate. Anal Biochem 258(1):155–157
Meyer H-P, Turner NJ (2009) Biotechnological manufacturing options for organic chemistry. Mini Rev Org Chem 6(4):300–306
Muhr AH, Blanshard JMV (1982) Diffusion in gels. Polym 23(7):1012–1026
Murray I, Williams PC (2001) Near-infrared technology in the agricultural and food industries. Chemical principles of near-IR technologies, 2nd edn. American Association of Cereal Chemists, St. Paul
Panula-Perälä J, Šiurkus J, Vasala A, Wilmanowski R, Casteleijn MG, Neubauer P (2008) Enzyme controlled glucose auto-delivery for high cell density cultivations in microplates and shake flasks. Microb Cell Fact 7:31
Riesenberg D (1991) High-cell-density cultivation of E. coli. Curr Opin Biotechnol 2(3):380–384
Samorski M, Müller-Newen G, Büchs J (2005) Quasi-continuous combined scattered light and fluorescence measurements: a novel measurement technique for shaken microtiter plates. Biotechnol Bioeng 92(1):61–68
Scheidle M, Dittrich B, Klinger J, Ikeda H, Klee D, Büchs J (2011) Controlling pH in shake flasks using polymer-based controlled-release discs with pre-determined release kinetics. BMC Biotechnol 11:25
Scheidle M, Jeude M, Dittrich B, Denter S, Kensy F, Suckow M, Klee D, Büchs J (2010) High-throughput screening of H. polymorpha clones in the batch compared with the controlled-release fed-batch mode on a small scale. FEMS Yeast Res 10(1):83–92
Scheidle M, Klinger J, Büchs J (2007) Combination of on-line pH and oxygen transfer rate measurement in shake flasks by fiber optical technique and respiration activity monitoring system (RAMOS). Sensors 7(12):3472–3480
Stöckmann C, Losen M, Dahlems U, Knocke C, Gellissen G, Büchs J (2003) Effect of oxygen supply on passaging, stabilising and screening of recombinant H. polymorpha production strains in test tube cultures. FEMS Yeast Res 4(2):195–205
Stöckmann C, Maier U, Anderlei T, Knocke C, Gellissen G, Büchs J (2003) The oxygen transfer rate as key parameter for the characterization of H. polymorpha screening cultures. J Ind Microbiol Biotechnol 30(10):613–622
Stöckmann C, Scheidle M, Dittrich B, Merckelbach A, Hehmann G, Melmer G, Klee D, Büchs J, Kang HA, Gellissen G (2009) Process development in H. polymorpha and Arxula adeninivorans, a re-assessment. Microb Cell Fact 8:22
Valgepea K, Adamberg K, Nahku R, Lahtvee P-J, Arike L, Vilu R (2010) Systems biology approach reveals that overflow metabolism of acetate in E. coli is triggered by carbon catabolite repression of acetyl-CoA synthetase. BMC Syst Biol 4:166
Van Wijk R (1968) Alpha-glucosidase synthesis respiratory enzymes and catabolite repression in yeast 1. Effects of glucose and maltose on inducible alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Proc K Ned Akad Wet C 71(1):60–71
Weuster-Botz D (2005) Parallel reactor systems for bioprocess development. In: Technology Transfer in Biotechnology: From Lab to Industry to Production, vol 92. Advances in biochemical engineering-biotechnology. Springer, Berlin Heidelberg New York, pp 125–143
Weuster-Botz D, Altenbach-Rehm J, Arnold M (2001) Parallel substrate feeding and pH-control in shaking flasks. Biochem Eng J 7(2):163–170
Xu B, Jahic M, Blomsten G, Enfors SO (1999) Glucose overflow metabolism and mixed-acid fermentation in aerobic large-scale fed-batch processes with E. coli. Appl Microbiol Biotechnol 51(5):564–571
Yankov D (2004) Diffusion of glucose and maltose in polyacrylamide gel. Enzyme Microb Technol 34(6):603–610
Zhang YK, Taiming L, Liu JJ (2003) Low temperature and glucose enhanced T7 RNA polymerase-based plasmid stability for increasing expression of glucagon-like peptide-2 in E. coli. Prot Expr Purif 29(1):132–139
Zhang Z, Perozziello G, Boccazzi P, Sinskey AJ, Geschke O, Jensen KF (2007) Micro-bioreactors for bioprocess development. JALA 12(3):143–151
Acknowledgments
The E. coli BL21 (DE3) pRhotHi-2––Ec FbFP was kindly provided by T. Drepper, Institute of Molecular Enzyme Technology, Heinrich-Heine-University Düsseldorf/Germany. The H. polymorpha RB 11 pC10-FMD (pFMD-GFP) was kindly supplied by the company RheinBiotech. The authors thank the company Lohmann for the supply of adhesive foils and Mr. Gerhard Otto from the Fraunhofer ILT, RWTH Aachen University (Germany) for the modification of the adhesive foils. The authors also thank Mr. Alfons Will for preparing the technical drawings.
Conflict of interest
All authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wilming, A., Bähr, C., Kamerke, C. et al. Fed-batch operation in special microtiter plates: a new method for screening under production conditions. J Ind Microbiol Biotechnol 41, 513–525 (2014). https://doi.org/10.1007/s10295-013-1396-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-013-1396-x