Abstract
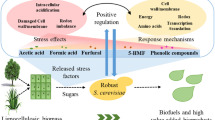
Bioethanol is an attractive alternative to fossil fuels. Saccharomyces cerevisiae is the most important ethanol producer. However, yeast cells are challenged by various environmental stresses during the industrial process of ethanol production. The robustness under heat, acetic acid, and furfural stresses was improved for ethanologenic S. cerevisiae in this work using genome shuffling. Recombinant yeast strain R32 could grow at 45°C, and resist 0.55% (v/v) acetic acid and 0.3% (v/v) furfural at 40°C. When ethanol fermentation was conducted at temperatures ranging from 30 to 42°C, recombinant strain R32 always gave high ethanol production. After 42 h of fermentation at 42°C, 187.6 ± 1.4 g/l glucose was utilized by recombinant strain R32 to produce 81.4 ± 2.7 g/l ethanol, which were respectively 3.4 and 4.1 times those of CE25. After 36 h of fermentation at 40°C with 0.5% (v/v) acetic acid, 194.4 ± 1.2 g/l glucose in the medium was utilized by recombinant strain R32 to produce 84.2 ± 4.6 g/l of ethanol. The extent of glucose utilization and ethanol concentration of recombinant strain R32 were 6.3 and 7.9 times those of strain CE25. The ethanol concentration produced by recombinant strain R32 was 8.9 times that of strain CE25 after fermentation for 48 h under 0.2% (v/v) furfural stress at 40°C. The strong physiological robustness and fitness of yeast strain R32 support its potential application for industrial production of bioethanol from renewable resources such as lignocelluloses.




Similar content being viewed by others
References
Almeida JRM, Modig T, Petersson A, Hahn-Hagerdal B, Liden G, Gorwa-Grauslund MF (2007) Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol 82:340–349
Balakumar S, Arasaratnam V, Balasubramaniam K (2001) Isolation and improvement of a thermotolerant Saccharomyces cerevisiae strain. World J Microbiol Biotechnol 17:739–746
de Virgillo C, Piper PW, Boller T, Wiemken A (1991) Acquisition of thermotolerance in Saccharomyces cerevisiae without heat shock proteins 104 and in the absence of protein synthesis. FEBS Lett 228:86–90
Eastmond DL, Nelson HC (2006) Genome-wide analysis reveals new roles for the activation domains of the Saccharomyces cerevisiae heat shock transcription factor (Hsf1) during the transient heat shock response. J Biol Chem 281:32909–32921
Ezeronye OU, Okerentugba PO (2001) Optimum conditions for yeast protoplast release and regeneration in Saccharomyces cerevisiae and Candida tropicalis using gut enzymes of the giant African snail Achatina achatina. Lett Appl Microbiol 32:190–193
Fujita Y, Ito J (2004) Synergistic saccharification, and direct fermentation to ethanol, of amorphous cellulose by use of an engineered yeast strain codisplaying three types of cellulolytic enzyme. Appl Environ Microbiol 70:1207–1212
Gera R, Dhamija SS, Gera T, Dalel S (1997) Intergeneric ethanol producing hybrids of thermotolerant Kluyveromyces and non-thermotolerant Saccharomyces cerevisiae. Biotechnol Lett 19:189–19313
He XP, Huai WH, Tie CJ, Liu YF, Zhang BR (2000) Breeding of high ergosterol-producing yeast srains. J Ind Microbiol Biotechnol 25:39–44
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci U S A 103:11206–11210
Hou L (2009) Novel methods of genome shuffling in Saccharomyces cerevisiae. Biotechnol Lett 31:671–677
Hou L (2010) Improved production of ethanol by novel genome shuffling in Saccharomyces cerevisiae. Appl Biochem Biotechnol 160:1084–1093
John RP, Gangadharan D, Nampoothiri KM (2008) Genome shuffling of Lactobacillus delbrueckii mutant and Bacillus amyloliquefaciens through protoplasmic fusion. Bioresour Technol 99:8008–8015
Keating JD, Panganiban C, Mansfield SD (2006) Tolerance and adaptation of ethanologenic yeasts to lignocellulosic inhibitory compounds. Biotechnol Bioeng 93:1196–1206
Krisch J, Szajáni B (1997) Ethanol and acetic acid tolerance in free and immobilized cells of Saccharomyces cerevisiae and Acetobacter aceti. Biotechnol Lett 19:525–528
Lewis JG, Learmonth RP, Watson K (1993) The role of growth phase and ethanol in freeze-thaw stress resistance of Saccharomyces cerevisiae. Appl Environ Microbiol 59:1065–1071
Lin FM, Qiao B, Yuan YJ (2009) Comparative proteomic analysis of tolerance and adaptation of ethanologenic Saccharomyces cerevisiae to furfural, a lignocellulosic inhibitory compound. Appl Environ Microbiol 75:3765–3776
Lindén T, Peetre J, Hahn-Hägerdal B (1992) Isolation and characterization of acetic acid-tolerant galactose fermenting strains of Saccharomyces cerevisiae from a spent sulfite liquor fermentation plant. Appl Environ Microbiol 58:1661–1669
Mager WH, Ferreir PM (1993) Stress response of yeast. Biochem J 290:1–13
Margeot A, Hahn-Hagerdal B, Edlund M, Slade R, Monot F (2009) New improvements for lignocellulosic ethanol. Curr Opin Biotechnol 20:372–380
Paiva S, Althoff S, Casal M, Leão C (1999) Transport acetate in mutants of Saccharomyces cerevisiae defective in monocarboxylate permeases. FEMS Microbiol Lett 170:301–306
Patnaik R, Louie S, Gavrilovic V, Perry K, Stemmer WPC, Ryan CM, del Cardayré S (2002) Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20:707–712
Phowchinda O, Delia-Dupuy ML, Strehaiano P (1995) Effects of acetic acid on growth and fermenting activity of Saccharomyces cerevisiae. Biotechnol Lett 17:237–242
Rajoka MI, Ferhan M, Khalid AM (2005) Kinetics and thermodynamics of ethanol production by a thermotolerant mutant of Saccharomyces cerevisiae in a microprocessor-controlled bioreactor. Lett Appl Microbiol 40:316–321
Shi D, Wang C, Wang K (2009) Genome shuffling to improve thermotolerance, ethanol tolerance and ethanol productivity of Saccharomyces cerevisiae. J Ind Microbiol Biotechnol 36:139–147
Sridhar M, Sree NK, Rao LV (2002) Effect of UV radiation on thermotolerance ethanol tolerance and osmotolerance of Saccharomyces cerevisiae VS1 and VS3 strains. Bioresour Technol 83:199–202
Trevisol ETV, Panek AD, Mannarino SC, Eleutherio ECA (2011) The effect of trehalose on the fermentation performance of aged cells of Saccharomyces cerevisiae. Appl Microbiol Biotechnol 90:697–704
Wei P, Li Z, Lin Y, He P, Jiang N (2007) Improvement of the multiple-stress tolerance of an ethanologenic Saccharomyces cerevisiae strain by freeze-thaw treatment. Biotechnol Lett 29:1501–1508
Wei P, Li Z, He P, Lin Y, Jiang N (2008) Genome shuffling in the ethanologenic yeast Candida krusei to improve acetic acid tolerance. Biotechnol Appl Biochem 49:113–120
Yamamoto N, Maeda Y, Ikeda A, Sakurai H (2008) Regulation of thermotolerance by stress-induced transcription factors in Saccharomyces cerevisiae. Eukaryot Cell 7:783–790
Zhang JG, Liu XY, He XP, Guo XN, Lu Y, Zhang BR (2010) Improvement of acetic acid tolerance and fermentation performance of diploid ethanologenic yeast by disruption of the FPS1 aquaglyceroporin gene. Biotechnol Lett 33:277–284
Zhang YX, Perry K, Vinci VA, Powell K, Stemmer WPC, del Cardayré SB (2002) Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature 415:644–646
Acknowledgments
The authors would like to acknowledge the financial support of Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX1-YW-11-C4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, Y., Cheng, YF., He, XP. et al. Improvement of robustness and ethanol production of ethanologenic Saccharomyces cerevisiae under co-stress of heat and inhibitors. J Ind Microbiol Biotechnol 39, 73–80 (2012). https://doi.org/10.1007/s10295-011-1001-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-1001-0