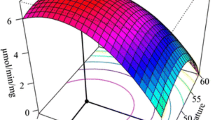
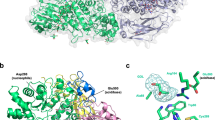
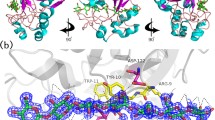
Abstract
β-d-Xylosidase/α-l-arabinofuranosidase from Selenomonas ruminantium is the most active enzyme reported for catalyzing hydrolysis of 1,4-β-d-xylooligosaccharides to d-xylose. One property that could use improvement is its relatively high affinities for d-glucose and d-xylose (K i ~ 10 mM), which would impede its performance as a catalyst in the saccharification of lignocellulosic biomass for the production of biofuels and other value-added products. Previously, we discovered that the W145G variant expresses K d-glucosei and K d-xylosei twofold and threefold those of the wild-type enzyme. However, in comparison to the wild type, the variant expresses 11% lower k d-xylobiosecat and much lower stabilities to temperature and pH. Here, we performed saturation mutagenesis of W145 and discovered that the variants express K i values that are 1.5–2.7-fold (d-glucose) and 1.9–4.6-fold (d-xylose) those of wild-type enzyme. W145F, W145L, and W145Y express good stability and, respectively, 11, 6, and 1% higher k d-xylobiosecat than that of the wild type. At 0.1 M d-xylobiose and 0.1 M d-xylose, kinetic parameters indicate that W145F, W145L, and W145Y catalytic activities are respectively 46, 71, and 48% greater than that of the wild-type enzyme.








Similar content being viewed by others
Abbreviations
- SXA:
-
β-d-Xylosidase/α-l-arabinofuranosidase from Selenomonas ruminantium
- 4NPX:
-
4-nitrophenyl β-d-xylopyranoside
- 4NPA:
-
4-nitrophenyl α-l-arabinofuranoside
- MUX:
-
4-methylumbelliferyl β-d-xylopyranoside
- X2:
-
D-xylobiose
- SXA-C3:
-
SXA containing these mutations W145G, T265A, P328L, and N516D
References
Adelsberger H, Hertel C, Glawischnig E, Zverlov VV, Schwarz WH (2004) Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150:2257–2266
Biarnés X, Nieto J, Planas A, Rovira C (2006) Substrate distortion in the Michaelis complex of bacillus 1, 3–1, 4-beta-glucanase. Insight from first principles molecular dynamics simulations. J Biol Chem 281:1432–1441
Brunzelle JS, Jordan DB, McCaslin DR, Olczak A, Wawrzak Z (2008) Structure of the two-subsite beta-d-xylosidase from Selenomonas ruminantium in complex with 1, 3-bis[Tris(hydroxymethyl)methylamino]propane. Arch Biochem Biophys 474:157–166
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2008) The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37:D233–D238
Davies GJ, Mackenzie L, Varrot A, Dauter M, Brzozowski AM, Scülein M, Withers SG (1998) Snapshots along an enzymatic reaction coordinate: analysis of a retaining beta-glycoside hydrolase. Biochemistry 37:11707–11713
Durette PL, Horton D (1971) Conformational analysis of sugars and their derivatives. Adv Carbohydr Chem Biochem 26:49–125
Fan Z, Yuan L, Jordan DB, Wagschal K, Heng C, Braker JD (2010) Engineering lower inhibitor affinities in beta-d-xylosidase. Appl Microbiol Biotechnol 86:1099–1113
Fauchère J-L, Pliška V (1983) Hydrophobic parameters pi. of amino acid side chains from the partitioning of N-acetyl-amino acid amides. Eur J Med Chem 18:369–375
Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182:319–326
Gong CS, Cao NJ, Du J, Tsao GT (1999) Ethanol production from renewable resources. Adv Biochem Eng Biotechnol 65:207–241
Gray KA, Zhao L, Emptage M (2006) Bioethanol. Curr Opin Chem Biol 10:141–146
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Johnson KA, Simpson ZB, Blom T (2009) Global kinetic explorer: a new computer program for dynamic simulation and fitting of kinetic data. Anal Biochem 387:20–29
Jordan DB, Dien BS, Li X-L, Cotta MA (2006) Hemicellulases for mediating biomass saccharification. In: Renewable Energy 2006 Committee (ed) Proceedings of renewable energy. Japan, pp 1036–1041
Jordan DB, Li X-L, Dunlap CA, Whitehead TR, Cotta MA (2007) Beta-d-xylosidase from Selenomonas ruminantium of glycoside hydrolase family 43. Appl Biochem Biotechnol 137–140:93–104
Jordan DB, Li X-L, Dunlap CA, Whitehead TR, Cotta MA (2007) Structure-function relationships of a catalytically efficient beta-d-xylosidase. Appl Biochem Biotechnol 141:51–76
Jordan DB, Li X-L (2007) Variation in relative substrate specificity of bifunctional beta-d-xylosidase/alpha-l-arabinofuranosidase by single-site mutations: roles of substrate distortion and recognition. Biochim Biophys Acta 1774:1192–1198
Jordan DB, Braker JD (2007) Inhibition of the two-subsite beta-d-xylosidase from Selenomonas ruminantium by sugars: competitive, noncompetitive, double binding, and slow binding modes. Arch Biochem Biophys 465:231–246
Jordan DB (2008) Beta-d-xylosidase from Selenomonas ruminantium: catalyzed reactions with natural and artificial substrates. Appl Biochem Biotechnol 146:137–149
Jordan DB, Braker JD (2009) Beta-d-xylosidase from Selenomonas ruminantium: thermodynamics of enzyme-catalyzed and noncatalyzed reactions. Appl Biochem Biotechnol 155:330–346
Jordan DB, Mertens JA, Braker JD (2009) Aminoalcohols as probes of the two-subsite active site of beta-d-xylosidase from Selenomonas ruminantium. Biochim Biophys Acta 1794:144–158
Jordan DB, Braker JD (2010) Beta-d-Xylosidase from Selenomonas ruminantium: role of glutamate 186 in catalysis revealed by site-directed mutagenesis, alternate substrates, and active-site inhibitor. Appl Biochem Biotechnol 161:395–410
Jordan DB, Wagschal K (2010) Properties and applications of microbial beta-d-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium. Appl Microbiol Biotechnol 86:1647–1658
Klinke HB, Thomsen AB, Ahring BK (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol 66:10–26
Larsson AM, Bergfors T, Dultz E, Irwin DC, Roos A, Driguez H, Wilson DB, Jones TA (2005) Crystal structure of Thermobifida fusca endoglucanase Cel6A in complex with substrate and inhibitor: the role of tyrosine Y73 in substrate ring distortion. Biochemistry 44:12915–12922
Larsson S, Palmqvist E, Hahn-Hägerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant NO (1999) The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159
Leatherbarrow RJ (2001) Grafit version 5. Erithacus Software Ltd., Horley
Lee YY, Iyer P, Torget RW (1999) Dilute-acid hydrolysis of lignocellulosic biomass. Adv Biochem Eng Biotechnol 65:93–115
Luo CD, Brink DL, Blanch HW (2002) Identification of potential fermentation inhibitors in conversion of hybrid poplar hydrolyzate to ethanol. Biomass Bioenergy 22:125–138
Notenboom V, Birsan C, Nitz M, Rose DR, Warren RA, Withers SG (1998) Insights into transition state stabilization of the beta-1, 4-glycosidase Cex by covalent intermediate accumulation in active site mutants. Nat Struct Mol Biol 5:812–818
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Bioresour Technol 74:25–33
Pattanaik S, Huan CH, Yuan L (2008) The interaction domains of the plant Myc-like bHLH transcription factors can regulate the transactivation strength. Planta 227:707–715
Saha BC (2003) Hemicellulose bioconversion. J Ind Microbiol Biotechnol 30:279–291
Sulzenbacher G, Driguez H, Henrissat B, Scülein M, Davies GJ (1996) Structure of the Fusarium oxysporum endoglucanase I with a nonhydrolyzable substrate analogue: substrate distortion gives rise to the preferred axial orientation for the leaving group. Biochemistry 35:15280–15287
Tews I, Perrakis A, Oppenheim A, Dauter Z, Wilson KS, Vorgias CE (1996) Bacterial chitobiase structure provides insight into catalytic mechanism and the basis of Tay–Sachs disease. Nat Struct Mol Biol 3:638–648
Vasella A, Davies GJ, Böhm M (2002) Glycosidase mechanisms. Curr Opin Chem Biol 6:619–629
Wagschal K, Heng C, Lee CC, Robertson GH, Orts WJ, Wong DWS (2009) Purification and characterization of a glycoside hydrolase family 43 beta-xylosidase from Geobacillus thermoleovorans IT-08. Appl Biochem Biotechnol 155:304–313
Wimley WC, Creamer TP, White SH (1996) Solvation energies of amino acid side chains and backbone in a family of host-guest pentapeptides. Biochemistry 35:5109–5124
Author information
Authors and Affiliations
Corresponding author
Additional information
The mention of firm names or trade products does not imply that they are endorsed or recommended by the US Department of Agriculture over other firms or similar products not mentioned.
Rights and permissions
About this article
Cite this article
Jordan, D.B., Wagschal, K., Fan, Z. et al. Engineering lower inhibitor affinities in β-d-xylosidase of Selenomonas ruminantium by site-directed mutagenesis of Trp145. J Ind Microbiol Biotechnol 38, 1821–1835 (2011). https://doi.org/10.1007/s10295-011-0971-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-011-0971-2