Abstract
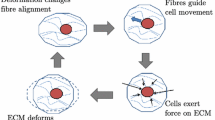
Physical cues play a fundamental role in a wide range of biological processes, such as embryogenesis, wound healing, tumour invasion and connective tissue morphogenesis. Although it is well known that during these processes, cells continuously interact with the local extracellular matrix (ECM) through cell traction forces, the role of these mechanical interactions on large scale cellular and matrix organization remains largely unknown. In this study, we use a simple theoretical model to investigate cellular and matrix organization as a result of mechanical feedback signals between cells and the surrounding ECM. The model includes bi-directional coupling through cellular traction forces to deform the ECM and through matrix deformation to trigger cellular migration. In addition, we incorporate the mechanical contribution of matrix fibres and their reorganization by the cells. We show that a group of contractile cells will self-polarize at a large scale, even in homogeneous environments. In addition, our simulations mimic the experimentally observed alignment of cells in the direction of maximum stiffness and the building up of tension as a consequence of cell and fibre reorganization. Moreover, we demonstrate that cellular organization is tightly linked to the mechanical feedback loop between cells and matrix. Cells with a preference for stiff environments have a tendency to form chains, while cells with a tendency for soft environments tend to form clusters. The model presented here illustrates the potential of simple physical cues and their impact on cellular self-organization. It can be used in applications where cell-matrix interactions play a key role, such as in the design of tissue engineering scaffolds and to gain a basic understanding of pattern formation in organogenesis or tissue regeneration.








Similar content being viewed by others
References
Barocas VH, Tranquillo RT (1997a) An anisotropic biphasic theory of tissue-equivalent mechanics: the interplay among cell traction, fibrillar network deformation, fibril alignment, and cell contact guidance. J Biomech Eng Trans Asme 119(2):137–145
Barocas VH, Tranquillo RT (1997b) A finite element solution for the anisotropic biphasic theory of tissue-equivalent mechanics: the effect of contact guidance on isometric cell traction measurement. J Biomech Eng 119(3):261–268
Bauer AL, Jackson TL et al (2009) Topography of extracellular matrix mediates vascular morphogenesis and migration speeds in angiogenesis. PLoS Comput Biol 5(7):e1000445
Bell E, Ivarsson B et al (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 76(3):1274–1278
Bischofs IB, Schwarz US (2003) Cell organization in soft media due to active mechanosensing. Proc Natl Acad Sci USA 100(16):9274–9279
Bischofs IB, Schwarz US (2006) Collective effects in cellular structure formation mediated by compliant environments: a Monte Carlo study. Acta Biomater 2(3):253–265
Boerboom RA, Driessen NJB et al (2003) Finite element model of mechanically induced collagen fiber synthesis and degradation in the aortic valve. Ann Biomed Eng 31(9):1040–1053
Borau C, Kamm RD et al (2011) Mechano-sensing and cell migration: a 3D model approach. Phys Biol 8(6):066008
Califano JP, Reinhart-King CA (2010) Substrate stiffness and cell area predict cellular traction stresses in single cells and cells in contact. Cell Mol Bioeng 3(1):68–75
Checa S, Prendergast PJ (2009) A mechanobiological model for tissue differentiation that includes angiogenesis: a lattice-based modeling approach. Ann Biomed Eng 37(1):129–145
Choquet D, Felsenfeld DP et al (1997) Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88(1):39–48
Dallon JC, Sherratt JA et al (1999) Mathematical modelling of extracellular matrix dynamics using discrete cells: fiber orientation and tissue regeneration. J Theor Biol 199(4):449–471
Delvoye P, Wiliquet P et al (1991) Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol 97(5):898–902
Discher DE, Janmey P et al (2005) Tissue cells feel and respond to the stiffness of their substrate. Science 310(5751):1139–1143
Dokukina IV, Gracheva ME (2010) A model of fibroblast motility on substrates with different rigidities. Biophys J 98(12):2794– 2803
Driessen NJB, Mol A et al (2007) Modeling the mechanics of tissue-engineered human heart valve leaflets. J Biomech 40(2):325–334
Ehrlich HP, Rajaratnam JB (1990) Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 22(4):407–417
Engler AJ, Sen S et al (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Farge E (2003) Mechanical induction of twist in the Drosophila foregut/stomodeal primordium. Curr Biol 13(16):1365–1377
Freyman TM, Yannas IV, Yokoo R, Gibson LJ (2001) Fibroblast contraction of a collagen-GAG matrix. Biomaterials 22(21):2883–2891
Galbraith CG, Yamada KM et al (2002) The relationship between force and focal complex development. J Cell Biol 159(4):695–705
Ghibaudo M, Saez A et al (2008) Traction forces and rigidity sensing regulate cell functions. Soft Matter 4(9):1836–1843
Gilbert TW, Wognum S et al (2008) Collagen fiber alignment and biaxial mechanical behavior of porcine urinary bladder derived extracellular matrix. Biomaterials 29(36):4775–4782
Gov NS (2009) Traction forces during collective cell motion. HFSP J 3(4):223–227
Grinnell F, Lamke CR (1984) Reorganization of hydrated collagen lattices by human skin fibroblasts. J Cell Sci 66:51–63
Guo WH, Frey MT et al (2006) Substrate rigidity regulates the formation and maintenance of tissues. Biophys J 90(6):2213–2220
Hadjipanayi E, Mudera V et al (2009) Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med 3(2):77–84
Hall BK, Miyake T (1995) Divide, accumulate, differentiate: Cell condensation in skeletal development revisited. Int J Dev Biol 39(6):881–893
Hariton I, deBotton G et al (2007) Stress-driven collagen fiber remodeling in arterial walls. Biomech Model Mechanobiol 6(3):163– 175
Harris AK, Stopak D et al (1981) Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290(5803):249–251
Holzapfel GA, Gasser TC (2001) A viscoelastic model for fiber-reinforced composites at finite strains: continuum basis, computational aspects and applications. Comput Methods Appl Mech Eng 190(34):4379–4403
Holzapfel GA, Gasser TC et al (2002) A structural model for the viscoelastic behavior of arterial walls: continuum formulation and finite element analysis. Eur J Mech A Solids 21(3):441–463
Huang D, Chang TR et al (1993) Mechanisms and dynamics of mechanical strengthening in ligament-equivalent fibroblast-populated collagen matrices. Ann Biomed Eng 21(3):289–305
Hutson MS, Ma X (2008) Mechanical aspects of developmental biology: perspectives on growth and form in the (post)-genomic age. Preface. Phys Biol 5(1):015001
Ingber DE (2008) Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol 18(5):356–364
Jeon H, Kim E, Grigoropoulos CP (2011) Measurement of contractile forces generated by individual fibroblasts on self-standing fiber scaffolds. Biomed Microdevices 13(1):107–115
Johnson AW, Harley BA (eds) (2011) Mechanbiology of cell-cell and cell-matrix interactions. Springer, New York
Jungbauer S, Gao HJ et al (2008) Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J 95(7):3470–3478
Kirmse R, Otto H et al (2011) Interdependency of cell adhesion, force generation and extracellular proteolysis in matrix remodeling. J Cell Sci 124(Pt 11):1857–1866
Klebe RJ, Caldwell H et al (1989) Cells transmit spatial information by orienting collagen fibers. Matrix 9(6):451–458
Kostyuk O, Brown RA (2004) Novel spectroscopic technique for in situ monitoring of collagen fibril alignment in gels. Biophys J 87(1):648–655
Kuhl E, Holzapfel GA (2007) A continuum model for remodeling in living structures. J Mater Sci 42(21):8811–8823
Lee J, Leonard M et al (1994) Traction forces generated by locomoting keratocytes. J Cell Biol 127(6):1957–1964
Levayer R, Lecuit T (2012) Biomechanical regulation of contractility: spatial control and dynamics. Trends Cell Biol 22(2):61–81
Lo CM, Wang HB et al (2000) Cell movement is guided by the rigidity of the substrate. Biophys J 79(1):144–152
Marinkovic A, Mih JD et al (2012) Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-beta responsiveness. Am J Physiol Lung Cell Mol Physiol 303(3):L169–180
Maruthamuthu V, Sabass B et al (2011) Cell-ECM traction force modulates endogenous tension at cell–cell contacts. Proc Natl Acad Sci USA 108(12):4708–4713
McDougall S, Dallon J et al (2006) Fibroblast migration and collagen deposition during dermal wound healing: mathematical modelling and clinical implications. Philos Trans A Math Phys Eng Sci 364(1843):1385–1405
Mitrossilis D, Fouchard J et al (2009) Single-cell response to stiffness exhibits muscle-like behavior. Proc Natl Acad Sci USA 106(43):18243–18248
Mitrossilis D, Fouchard J et al (2010) Real-time single-cell response to stiffness. Proc Natl Acad Sci USA 107(38):16518–16523
Murray JD, Oster GF (1984) Cell traction models for generating pattern and form in morphogenesis. J Math Biol 19(3):265–279
Murray JD, Oster GF et al (1983) A mechanical model for mesenchymal morphogenesis. J Math Biol 17(1):125–129
Oster GF, Murray JD et al (1983) Mechanical aspects of mesenchymal morphogenesis. J Embryol Exp Morphol 78:83–125
Paszek MJ, Zahir N et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Pelham RJ Jr, Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA 94(25):13661–13665
Petroll WM (2007) Dynamic assessment of cell-matrix mechanical interactions in three-dimensional culture. Methods Mol Biol 370: 67–82
Riveline D, Zamir E et al (2001) Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153(6):1175–1185
Saez A, Buguin A et al (2005) Is the mechanical activity of epithelial cells controlled by deformations or forces? Biophys J 89(6):L52–54
Schluter DK, Ramis-Conde I et al (2012) Computational modeling of single-cell migration: the leading role of extracellular matrix fibers. Biophys J 103(6):1141–1151
Schwarz US, Safran SA (2002) Elastic interactions of cells. Phys Rev Lett 88(4):048102
Stopak D, Harris AK (1982) Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev Biol 90(2):383–398
Takakuda K, Miyairi H (1996) Tensile behaviour of fibroblasts cultured in collagen gel. Biomaterials 17(14):1393–1397
Thiery JP (1984) Mechanisms of cell migration in the vertebrate embryo. Cell Differ 15(1):1–15
Tranquillo RT, Durrani MA et al (1992) Tissue engineering science: consequences of cell traction force. Cytotechnology 10(3):225–250
Trichet L, Le Digabel J et al (2012) Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc Natl Acad Sci USA 109(18):6933–6938
Vogel V, Sheetz M (2006) Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 7(4):265–275
Wilson W, Driessen NJB et al (2006) Prediction of collagen orientation in articular cartilage by a collagen remodeling algorithm. Osteoarthr Cartil 14(11):1196–1202
Wozniak MA, Chen CS (2009) Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol 10(1):34–43
Zahm JM, Kaplan H et al (1997) Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskelet 37(1):33–43
Zulliger MA, Fridez P et al (2004) A strain energy function for arteries accounting for wall composition and structure. J Biomech 37(7):989–1000
Acknowledgments
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; DU298/14-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Checa, S., Rausch, M.K., Petersen, A. et al. The emergence of extracellular matrix mechanics and cell traction forces as important regulators of cellular self-organization. Biomech Model Mechanobiol 14, 1–13 (2015). https://doi.org/10.1007/s10237-014-0581-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-014-0581-9