Abstract
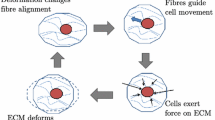
Blood and tissue cells mechanically interact with soft tissues and tissue-equivalent reconstituted collagen gels in a variety of situations relevant to biomedicine and biotechnology. A key phenomenon in these interactions is the exertion of traction force by cells on local collagen fibers which typically constitute the solid network of these tissues and gels and impart gross mechanical integrity. Two important consequences of cells exerting traction on such collagen networks are first, when the cells co-ordinate their traction, resulting in cell migration, and second, when their traction is sufficient to deform the network. Such cell-collagen network interactions are coupled in a number of ways. Network deformation, for example, can result in net alignment of collagen fibers, eliciting contact guidance, wherein cells move with bidirectional bias along an axis of fiber alignment, potentially leading to a nonuniform cell distribution. This may govern cell accumulation in wounds and be exploited to control cell infiltration of bioartificial tissues and organs. Another consequence of cell traction is the resultant stress and strain in the network which modulate cell protein and DNA synthesis and differentiation. We summarize, here, relevant mathematical theories which we have used to describe the inherent coupling of cell dynamics and tissue mechanics in cell-populated collagen gels via traction. The development of appropriate models based on these theories, in an effort to understand how events in wound healing govern the rate and extent of wound contraction, and to measure cell traction forces in vitro, are described. Relevant observations and speculation from cell biology and medicine that motivate or serve to critique the assumptions made in the theories and models are also summarized.
Similar content being viewed by others
Abbreviations
- ECM:
-
Extracellular Matrix
- FPCL:
-
Fibroblast-Populated Collagen Lattice
- FPCM:
-
Fibroblast-Populated Collagen Microsphere
References
BellE (1983) The reconstitution of living skin. J. Invest. Dermatol. 81: 2s-10s.
BellE, IvarssonB and MerrillC (1979) Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 76: 1274–1278.
BellowsCG, MelcherAH and AubinJE (1981) Contraction and organization of collagen gels by cells cultured from periodontal ligament, gingiva and bone suggest functional differences between cell types. J. Cell. Sci. 50: 299–314.
BellowsCG, MelcherAH, BhargavaU and AubinJE (1982) Fibroblasts contracting three-dimensional collagen gels exhibit ultrastructure consistent with either contraction or protein secretion. J. Ultrastr. Res. 78: 178–192.
BowenRM (1976) Theory of mixtures. In: EringenAC (ed) Continuum Physics. Academic Press, New York, pp. 1–127.
BrandleyBK and SchnaarRL (1989) Tumor cell haptotaxis on covalently immobilized linear and exponential gradients of a cell adhesion peptide. Dev. Biol. 135 (1): 74–86.
ButtleDJ and EhrlichHP (1983) Comparative studies of collagen lattice contraction utilizing a normal and a transformed cell line. J. Cell. Physiol. 116 (2): 159–166.
ClarkRAF (1988) Overview and general considerations of wound repair. In: ClarkRAF and HensonPM (eds) The Molecular and Cellular Biology of Wound Repair. Plenum Press. New York, pp. 3–33.
CraneRE, GreenAE and NaghdiPM (1970) A mixture of viscous and elastic materials with different constituent temperatures. Quart. J. Mech. Appl. Math. 23: 171–184.
CrankJ (1975) The Mathematics of Diffusion. Oxford University Press, Oxford.
DanowskiBA and HarrisAK (1988) Changes in fibroblast contractility, morphology, and adhesion in response to a phorbol ester tumor promoter. Exp. Cell. Res. 177 (1): 47–59.
DelvoyeP, NusgensB and LapiereCM (1983) The capacity of retracting a collagen matrix is lost by dermatosparactic skin fibroblasts. J. Invest. Dermatol. 81 (3): 267–270.
DelvoyeP, WiliquetP, LevequeJL, NusgensB and LapiereC (1991) Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J. Invest. Dermatol. 97: 898–902.
DemboM and HarlowF (1986) Cell motion, contractile networks, and the physics of interpenetrating reactive flow. Biophys. J. 50 (1): 109–121.
Dickinson RB and Tranquillo RT (1993a) Transport equations and indices for random and biased cell migration based on single cell properties. SIAM J. Appl. Math., submitted.
Dickinson RB and Tranquillo RT (1993b) A stochastic model for cell random motility and haptotaxis. J. Math. Biol., in press.
Drew DA (1971) Averaged field equations for two-phase media. Stud. Appl. Math. L (2): 133–166.
Drew DA and Segel LA (1971) Averaged equations for twophase flows. Stud. Appl. Math. L (3): 205–231.
DunnGA and EbendalT (1978) Contact guidance on oriented collagen gels. Exp. Cell. Res. 111: 475–479.
DvorakHF (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315 (26): 1650–1659.
EhrlichHP (1988) The role of connective tissue matrix in wound healing. Prog. Clin. Biol. Res. 266: 243–258.
EhrlichHP, GriswoldTR and RajaratnamJB (1986) Studies on vascular smooth muscle cells and dermal fibroblasts in collagen matrices. Effects of heparin. Exp. Cell. Res. 164 (1): 154–162.
EhrlichHP and RajaratnamJB (1990) Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell 22 (4): 407–417.
EisenbergSR and GrodzinskyAJ (1985) Swelling of articular cartilage and other connective tissues: electromechanochemical forces. J. Orthop. Res. 3 (2): 148–159.
ElsdaleT and BardJ (1972) Collagen substrata for studies on cell behavior. J. Cell. Biol. 54: 626–637.
FarquharT, DawsonPR and TorzilliPA (1990) A microstructural model for the anisotropic drained stiffness of articular cartilage. J. Biomech. Eng. 112 (4): 414–425.
GilleryP, MaquartFX and BorelJP (1986) Fibronectin dependence of the contraction of collagen lattices by human skin fibroblasts. Exp. Cell. Res. 167 (1): 29–37.
GreenAE and NaghdiPM (1970) The flow of fluid through an elastic solid. Acta Mech. 9: 329–340.
GrinnellF and LamkeCR (1984) Reorganization of hydrated collagen lattices by human skin fibroblasts. J. Cell. Sci. 66: 51–63.
GrodzinskyAJ (1983) Electromechanical and physicochemical properties of connective tissue. Crit. Rev. Biomed. Eng. 9 (2): 133–199.
GuberS and RudolphR (1978) The myofibroblast. Surg. Gynecol. Obstet. 146: 641–649.
Guido S and Tranquillo RT (1993) A novel methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. (Accepted)
GuidryC and GrinnellF (1985) Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J. Cell Sci. 79: 67–81.
GuidryC and GrinnellF (1986) Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Collagen Rel. Res. 6: 515–529.
GuidryC and GrinnellF (1987) Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. J. Cell Biol. 104 (4): 1097–1103.
HarrisAK (1973) Behaviour of cultured cells on substrata of variable adhesiveness. Exp. Cell Res. 77: 285–297.
HarrisAK (1982) Traction and its relations to contraction in tissue cell locomotion. In: BellairsR, CurtisA and DunnG (eds) Cell Behaviour. Cambridge University Press. New York, pp. 77–108.
HarrisAK (1984) Tissue culture cells on deformable substrata: biomechanical implications. J. Biomech. Eng. 106: 19–24.
HarrisAK, StopakD and WarnerP (1984) Generation of spatially periodic patterns by a mechanical instability: a mechanical alternative to the Turing model. J. Embryol. Exp. Morphol. 80: 1–20.
HarrisAK, StopakD and WildP (1981) Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290 (5803): 249–251.
HarrisAK, WildP and StopakD (1980) Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science 208 (4440): 177–179.
HolmesMH (1986) Finite deformation of soft tissue: analysis of a mixture model in uni-axial compression. J. Biomech. Eng. 108 (4): 372–381.
HuWS, NybergSL, ShatfordRA and CerraRB (1991) Cultivation of hepatocytes in a new entrapment bioreactor: a potential bioartificial liver. In: SasakiR and IkuraK (eds) Animal Cell Culture and Production of Biologicals. Kluwer Academic Publishers, Dordrecht, pp. 75–80.
JainMK, BergRA and TandonGP (1990) Mechanical stress and cellular metabolism in living soft tissue composites. Biomaterials 11 (7): 465–472.
KenyonDE (1979) Consolidation in transversely isotropic solids. J. Appl. Mech. 46: 65–70.
KolodneyMS and WysolmerskiRB (1992) Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J. Cell Biol. 117: 73–82.
KwanMK, LaiWM and MowVC (1990) A finite deformation theory for cartilage and other soft hydrated connective tissues — I. Equilibrium results. J. Biomech. 23 (2): 145–155.
LandauLD and LifshitzEM (1970) Theory of Elasticity. Pergamon Press, New York.
LeaderWM, StopakD and HarrisAK (1983) Increased contractile strength and tightened adhesions to the substratum result from reverse transformation of CHO cells by dibutyryl cyclic adenosine monophosphate. J. Cell Sci. 64: 1–11.
LinCC and SegelLA (1974) Mathematics Applied to Deterministic Problems in the Natural Sciences. Macmillan Publishing Co., Inc., New York.
MadriJA and PrattBM (1986) Endothelial cell-matrix interactions: in vitro models of angiogenesis. J. Histochem. Cytochem. 34 (1): 85–91.
MakAF (1986) The apparent viscoelastic behavior of articular cartilage — the contributions from the intrinsic matrix viscoelasticity and interstitial fluid flows. J. Biomech. Eng. 108: 123–130.
MansourJ and MowVC (1976) The permeability of articular cartilage under compressive strain and at high pressures. J. Bone Joint Surg. 58A: 509–516.
MastBA, HaynesJH, KrummelTM, DiegelmannRF and CohenIK (1992) In vivo degradation of fetal wound hyaluronic acid results in increased fibroplasia, collagen deposition and neovascularization. Plast. Reconstr. Surg. 89 (3): 503–509.
MatthesT and GrulerH (1988) Analysis of cell locomotion. Contact guidance of human polymorphonuclear leukocytes. Eur. Biophys. J. 15 (6): 343–357.
McCarthyJB, SasDF and FurchtLT (1988) Mechanisms of parenchymal cell migration into wounds. In: ClarkRAF and HensonPM (eds) The Molecular and Cellular Biology of Wound Repair. Plenum Press, New York.
MochitateK, PawelekP and GrinnellF (1991) Stress relaxation of contracted collagen gels: disruption of actin filament bundles, release of cell surface fibronectin, and down-regulation of DNA and protein synthesis. Exp. Cell Res. 193 (1): 198–207.
ModisL (1991) Organization of the Extracellular Matrix: A Polarization Microscopic Approach. CRC Press, Boca Raton.
MontandonD, D'andiranG and GabbianiG (1977) The Mechanism of Wound Contraction and Epithelialization. Clin. Plast. Surg. 4 (3): 325–346.
MontandonD, GabbianiG, RyanGB and MajnoG (1973) The contractile fibroblast: its relevance in plastic surgery. Plast. Reconstr. Surg. 52: 286–292.
MontesanoR and OrciL (1988) Transforming growth factor-β stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc. Natl. Acad. Sci. USA 85: 4894–4897.
MoonAG and TranquilloRT (1993a) The fibroblast-populated collagen microsphere assay of cell traction force — Part 1. Continuum Model. AIChE J. 39 (1): 163–177.
Moon AG and Tranquillo RT (1993b) The fibroblast-populated collagen microsphere assay of cell traction force — Part 2. Measurement of the cell traction parameter. Submitted.
MowVC, KueiSC, LaiWM and ArmstrongCG (1980) Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J. Biomech. Eng. 102: 73–84.
MowVC, KwanMK, LaiWM and HolmesMH (1986) A finite deformation theory for nonlinearly permeable soft hydrated biological tissues. In: Schmid-SchonbeinGW, WooSL-Y and ZweifachBW (eds) Frontiers in Biomechanics. Springer-Verlag, New York, pp. 153–179.
MurrayJD and OsterGF (1984) Cell traction models for generating pattern and form in morphogenesis. J. Math. Biol. 19 (3): 265–279.
MurrayJD, OsterGF and HarrisAK (1983) A mechanical model for mesenchymal morphogenesis. J. Math. Biol. 17 (1): 125–129.
NakagawaS, PawelekP and GrinnellF (1989) Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp. Cell Res. 182 (2): 572–582.
NeremRM and GirardPR (1990) Hemodynamic influences on vascular endothelial biology. Toxicol. Pathol. 18: 572–582.
NilssonK and MosbachK (1987) Immobilized animal cells. Dev. Biol. Stand. 66: 183–193.
NilssonK, ScheirerW, MertenOW, OstbergL, LiehlE, KatingerHWD and MosbachK (1983) Entrapment of animal cells for production of monoclonal antibodies and other biomolecules. Nature 302: 629–630.
NoblePB and ShieldsED (1989) Time-based changes in fibroblast three-dimensional locomotory characteristics and phenotypes. Exp. Cell. Biol. 57 (5): 238–245.
NollertMU, DiamondSL and McIntireLV (1991) Hydrodynamic shear stress and mass transport modulation of endothelial cell metabolism. Biotech. Bioeng. 38: 588–602.
NusgensB, MerrillC, LapiereC and BellE (1984) Collagen biosynthesis by cells in a tissue equivalent matrix in vitro. Collagen Rel. Res. 4 (5): 351–363.
OdellGM, OsterG, AlberchP and BurnsideB (1981) The mechanical basis of morphogenesis. I. Epithelial folding and invagination. Dev. Biol. 85 (2): 446–462.
OsterGF, MurrayJD and HarrisAK (1983) Mechanical aspects of mesenchymal morphogenesis. J. Embryol. Exp. Morphol. 78: 83–125.
RudolphR (1979) Location of the force of wound contraction. Surg., Gynecol. Obstet. 148: 547–551.
RudolphR (1980) Contraction and the control of contraction. World J. Surg. 4: 279–287.
SchererGW (1989) Mechanics of syneresis — I. Theory. J. Non-Cryst. Solids 108: 18–27.
SchorSL (1980) Cell proliferation and migration on collagen substrata in vitro. J. Cell. Sci. 41: 159–175.
Schwartz MH, Leo PH and Lewis JL (1993) A microstructural model of articular cartilage. J. Biomech. Eng. (accepted)
SeemayerTA, SchurchW and LagaceR (1981) Myofibroblasts in human pathology. Hum. Path. 12: 491–492.
SegelLA (1980) Mathematical Models in Molecular and Cellular Biology. Cambridge University Press, New York.
SkalliO and GabbianiG (1988) The biology of the myofibroblast relationship to wound contraction and fibrocontractive diseases. In: ClarkRAF and HensonPM (eds) The Molecular and Cellular Biology of Wound Repair. Plenum Press, New York, pp. 373–394.
StopakD and HarrisAK (1982) Connective tissue morphogenesis by fibroblast traction. I. Tissue culture observations. Dev. Biol. 90 (2): 383–398.
TozerenA and SkalakR (1988) Interaction of stress and growth in a fibrous tissue. J. Theor. Biol. 130: 337–350.
TranquilloRT and LauffenburgerDA (1991) The definition and measurement of cell migration coefficients. Lect. Notes Biomath. 89: 475–486.
TranquilloRT and MurrayJD (1992) Continuum model of fibroblast-driven wound contraction: inflammation-mediation. J. Theor. Biol., 158: 135–172.
TrinkausJ (1984) Cells into organs (2nd edn). Prentice-Hall, Englewood Cliffs.
YannasIV, BurkeJF, OrgillDP and SkrabutEM (1982) Wound tissue can utilize a polymeric template to synthesize a functional extension of skin. Science 215 (4529): 174–176.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tranquillo, R.T., Durrani, M.A. & Moon, A.G. Tissue engineering science: Consequences of cell traction force. Cytotechnology 10, 225–250 (1992). https://doi.org/10.1007/BF00146673
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00146673