Abstract
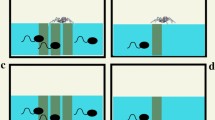
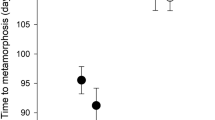
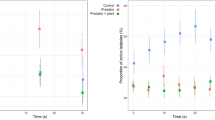
In aquatic ecosystems many biotic and abiotic factors influence predator–prey interactions directly or indirectly. In ephemeral pools the water level can change drastically, which may have a significant impact on predator–prey interactions, especially where ecological niches of predator(s) and prey comprise different layers of the water column. We tested the ability of benthic predatory Hoplobatrachus crassus tadpoles to detect their prey, tadpoles of the bottom-dweller Fejervarya syhadrensis and column-feeder Microhyla nilphamariensis, using visual and chemical cues, and the effect of a change in water level on the survival of the prey when exposed to H. crassus tadpoles. H. crassus tadpoles detected their prey using visual cues but not chemical ones (kairomones). Under the high-water-level condition, more M. nilphamariensis tadpoles survived than F. syhadrensis tadpoles. Under the low-water-level condition, predation levels did not differ for the two prey species. These results imply that H. crassus tadpoles hunt bottom-dwelling prey tadpoles using visual signals. M. nilphamariensis tadpoles escape the risk of predation from the bottom-dwelling predator mainly due to their ecological niche (the water column). These results showed that a change in water level can shift predation pressure between tadpoles residing in the different layers of a water column and determine the outcome of predator–prey interactions.


Similar content being viewed by others
References
Abrahams MV, Kattenfeld MG (1997) The role of turbidity as a constraint on predator-prey interactions in aquatic environments. Behav Ecol Sociobiol 40:169–174
Altig R, McDiarmid RW (1999) Diversity: familial and genetic characterization. In: McDiarmid RE, Altig R (eds) Tadpoles: the biology of anuran larvae. University of Chicago Press, Chicago, pp 295–335
Batabyal A, Gosavi SM, Gramapurohit NP (2014) Determining sensitive stages for learning to detect predators in larval bronzed frogs: importance of alarm cues in learning. J Biosci 39:701–710
Blaustein AR, Hatch A, Belden LK, Wildy EL (2001) Influence of abiotic and biotic factors on amphibians in ephemeral ponds with special reference to long-toed salamanders (Ambystoma macrodactylum). Isr J Zool 47:333–346
Van Buskirk J, Krügel A, Kunz J, Miss F, Stamm A (2014) The rate of degradation of chemical cues indicating predation risk: an experiment and review. Ethology 120:942–949
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5:338–352
Chivers DP, Dixson DL, White JR, Mc Cormick MI, Ferrari MC (2013) Degradation of chemical alarm cues and assessment of risk throughout the day. Ecol Evol 3:3925–3934
Dijkgraaf S (1963) The functioning and significance of the lateral-line organs. Biol Rev 38:51–105
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Fraker ME, Hu F, Cuddapah V et al (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529
Garg S, Das A, Kamei RG, Biju SD (2018) Delineating Microhyla ornata (Anura, Microhylidae): mitochondrial DNA barcodes resolve century-old taxonomic misidentification. Mitochondrial DNA Part B 3:856–861
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grosjean S, Vences M, Dubois A (2004) Evolutionary significance of oral morphology in the carnivorous tadpoles of tiger frogs, genus Hoplobatrachus (Ranidae). Biol J Linn Soc 81:171–181
Hettyey A, Rölli F, Thürlimann N, Zurcher A, Buskirk JV (2012) Visual cues contribute to predator detection in anuran larvae. Biol J Linn Soc 106:820–827
Hettyey A, Tóth Z, Thonhauser KE, Frommen JG, Penn DJ, Buskirk JV (2015) The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179:699–710
Heyer WR, McDiarmid RW, Weigmann DL (1975) Tadpoles, predation and pond habitats in the tropics. Biotropica 7:100–111
Hiragond NC, Saidapur SK (2001) Microhabitat choice of tadpoles of seven anuran species. Curr Herpetol 20:51–60
Kiesecker JM, Chivers DP, Blaustein AR (1996) The use of chemical cues in predator recognition by western toad tadpoles. Anim Behav 52:1237–1245
Klecka J, Boukal DS (2012) Who eats whom in a pool? A comparative study of prey selectivity by predatory aquatic insects. PLoS One 7:37741
Klecka J, Boukal DS (2014) The effect of habitat structure on prey mortality depends on predator and prey microhabitat use. Oecologia 176:183–191
Leahy SM, McCormick MI, Mitchell MD, Ferrari MCO (2011) To fear or to feed: the effects of turbidity on perception of risk by a marine fish. Biol Lett 7:811–813
Leu ST, Whiting MJ, Mahony MJ (2013) Making friends: social attraction in larval green and golden bell frogs Litoria aurea. PLoS One 8:56460
Middlemis Maher J, Werner EE, Denver RJ (2013) Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc R Soc B Biol Sci 280:20123075
Natale GS, Alcalde L, Herrera R, Cajade R, Schaefer EF, Marangoni F, Trudeau VL (2011) Underwater acoustic communication in the macrophagic carnivorous larvae of Ceratophrys ornata (Anura: Ceratophryidae). Acta Zool 92:46–53
Pohlmann K (2004) The importance of the lateral line in nocturnal predation of piscivorous catfish. J Exp Biol 207:2971–2978
Pohlmann K, Grasso FW, Breithaupt T (2001) Tracking wakes: the nocturnal predatory strategy of piscivorous catfish. Proc Natl Acad Sci 98:7371–7374
Polo-Cavia N, Boyero L, Martín-Beyer B, Barmuta L, Bosch J (2017) Joint effects of rising temperature and the presence of introduced predatory fish on montane amphibian populations. Anim Conserv 20:128–134
Pueta M, Perotti MG (2016) Anuran tadpoles learn to recognize injury cues from members of the same prey guild. Anim Cogn 19:745–751
Richter-Boix A, Llorente GA, Montori A (2007) A comparative study of predator-induced phenotype in tadpoles across a pond permanency gradient. Hydrobiologia 583:43–56
Saidapur S (2001) Behavioral ecology of anuran tadpoles: the Indian scenario. Proc Indian Natl Sci Acad B 67:311–322
Saidapur SK, Veeranagoudar DK, Hiragond NC, Shanbhag BA (2009) Mechanism of predator–prey detection and behavioral responses in some anuran tadpoles. Chemoecology 19:21–28
Schwalbe MAB, Webb JF (2014) Sensory basis for detection of benthic prey in two Lake Malawi cichlids. Zoology 117:112–121
Skelly DK (1997) Tadpole communities: Pond permanence and predation are powerful forces shaping the structure of tadpole communities. Am Sci 85:36–45
de So RO, Streicher JW, Sekonyela R, Forlani MC, Loader SP, Greenbaum E, Haddad CF (2012) Molecular phylogeny of microhylid frogs (Anura: Microhylidae) with emphasis on relationship among New World genera. BMC Evol Biol 12:241
Stauffer H-P, Semlitsch RD (1993) Effects of visual, chemical and tactile cues of fish on the behavioural responses of tadpoles. Anim Behav 46:355–364
Supekar SC, Gramapurohit NP (2017) Can embryonic skipper frogs (Euphlyctis cyanophlyctis) learn to recognise kairomones in the absence of a nervous system? J Biosci 42:459–468
Supekar SC, Gramapurohit NP (2018) Larval skipper frogs recognise kairomones of certain predators innately. J Ethol 36:143–149
Veeranagoudar D, Shanbhag B, Saidapur S (2004) Mechanism of food detection in the tadpoles of the bronze frog Rana temporalis. Acta Ethol 7:37–41
Wisenden BD (2000) Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc B Biol Sci 355:1205–1208
Wisenden BD (2003) Chemically mediated strategies to counter predation. In: Collin SP, Marshall NJ (eds) Sensory processing in aquatic environments. Springer, New York, pp 236–251
Acknowledgements
We thank Savitribai Phule Pune University for their permission to use the laboratory facilities of the Department of Zoology, and the Maharashtra State Biodiversity Board (MSBB), Nagpur for permission to collect and maintain the animals in a laboratory for experimentation (MSBB/Desk-5/Appl/NOC/CR-275/1362/2015–16). We are grateful to two anonymous reviewers for their valuable suggestions on the previous versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Ethical approval
The animal experimentation was performed following ethical guidelines established for animal usage by Savitribai Phule Pune University, Pune, India.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Phuge, S., Phuge, A. Predator-prey interactions of tadpoles in different layers of the water column. J Ethol 37, 197–202 (2019). https://doi.org/10.1007/s10164-019-00588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-019-00588-4