Abstract
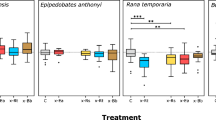
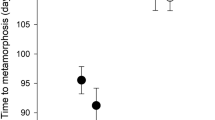
Chemical cues that evoke anti-predator developmental changes have received considerable attention, but it is not known to what extent prey use information from the smell of predators and from cues released through digestion. We conducted an experiment to determine the importance of various types of cues for the adjustment of anti-predator defences. We exposed tadpoles (common frog, Rana temporaria) to water originating from predators (caged dragonfly larvae, Aeshna cyanea) that were fed different types and quantities of prey outside of tadpole-rearing containers. Variation among treatments in the magnitude of morphological and behavioural responses was highly consistent. Our results demonstrate that tadpoles can assess the threat posed by predators through digestion-released, prey-borne cues and continually released predator-borne cues. These cues may play an important role in the fine-tuning of anti-predator responses and significantly affect the outcome of interactions between predators and prey in aquatic ecosystems. There has been much confusion regards terminology used in the literature, and therefore we also propose a more precise and consistent binomial nomenclature based on the timing of chemical cue release (stress-, attack-, capture-, digestion- or continually released cues) and the origin of cues (prey-borne or predator-borne cues). We hope that this new nomenclature will improve comparisons among studies on this topic.


Similar content being viewed by others
References
Brönmark C, Hansson L-A (2000) Chemical communication in aquatic systems. Oikos 88:103–109
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment. Fish Fish 4:227–234
Brown GE, Chivers DP, Smith RJF (1995) Localized defecation by pike: a response to labelling by cyprinid alarm pheromone? Behav Ecol Sociobiol 36:105–110
Chivers DP, Ferrari MCO (2013) Tadpole antipredator responses change over time: what is the role of learning and generalization? Behav Ecol 24:1114–1121
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator-prey systems: a review and prospectus. Ecoscience 5:338–352
Chivers DP, Wisenden BD, Smith RJF (1996) Damselfly larvae learn to recognize predators from chemical cues in the predator’s diet. Anim Behav 52:315–320
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
El-Balaa R, Blouin-Demers G (2013) Does exposure to cues of fish predators fed different diets affect morphology and performance of Northern Leopard Frog (Lithobates pipiens) larvae? Can J Zool 91:203–211
Ferland-Raymond B, March RE, Metcalfe CD, Murray DL (2010) Prey detection of aquatic predators: assessing the identity of chemical cues eliciting prey behavioral plasticity. Biochem Syst Ecol 38:169–177
Ferrari MCO, Trowell JJ, Brown GE, Chivers DP (2005) The role of learning in the development of threat-sensitive predator avoidance by fathead minnows. Anim Behav 70:777–784
Ferrari MCO, Brown MR, Pollock MS, Chivers DP (2007) The paradox of risk assessment: comparing responses of fathead minnows to capture-released and diet-released alarm cues from two different predators. Chemoecology 17:157–161
Ferrari MCO, Messier F, Chivers DP (2008) Degradation of chemical alarm cues under natural conditions: risk assessment by larval woodfrogs. Chemoecology 17:263–266
Fraker ME (2008) The dynamics of predation risk assessment: responses of anuran larvae to chemical cues of predators. J Anim Ecol 77:638–645
Fraker ME (2009) Predation risk assessment through chemical cues produced by multiple prey. Behav Ecol Sociobiol 63:1397–1402
Fraker ME, Hu F, Cuddapah V, McCollum SA, Relyea RA, Hempel J, Denver RJ (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529
Gonzalo A, Lopez P, Martin J (2007) Iberian green frog tadpoles may learn to recognize novel predators from chemical alarm cues of conspecifics. Anim Behav 74:447–453
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on their identification. Herpetologica 16:183–190
Harvell CD (1986) The ecology and evolution of inducible defences in a marine bryozoan: cues, costs and consequences. Am Nat 128:810–823
Harvell CD (1990) The ecology and evolution of inducible defences. Q Rev Biol 65:323–340
Hettyey A, Zsarnóczai S, Vincze K, Hoi H, Laurila A (2010) Interactions between the information content of different chemical cues affect induced defences in tadpoles. Oikos 119:1814–1822
Hettyey A, Vincze K, Zsarnóczai S, Hoi H, Laurila A (2011) Costs and benefits of defenses induced by predators differing in dangerousness. J Evol Biol 24:1007–1019
Hettyey A, Rölli F, Thürlimann N, Zürcher A-C, Van Buskirk J (2012) Visual cues contribute to predator detection in anuran larvae. Biol J Linn Soc 106:820–827
Hews DK (1988) Alarm response in larval western toads, Bufo boreas—release of larval chemicals by a natural predator and its effect on predator capture efficiency. Anim Behav 36:125–133
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signaling and predator labeling in the avoidance response of a marine gastropod. Oikos 104:43–50
Kats LB, Dill LM (1998) The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394
Kesavaraju B, Damal K, Juiano SA (2007) Threat-sensitive behavioral responses to concentrations of water-borne cues from predation. Ethology 113:119–206
Kiesecker JM, Chivers DP, Marco A, Quilchano C, Anderson MT, Blaustein AR (1999) Identification of a disturbance signal in larval red-legged frogs, Rana aurora. Anim Behav 57:1295–1300
Kishida O, Nishimura K (2005) Multiple inducible defences against multiple predators in the anuran tadpole, Rana pirica. Evol Ecol Res 7:619–631
LaFiandra EM, Babbitt KJ (2004) Predator induced phenotypic plasticity in the pinewoods tree frog, Hyla femoralis: necessary cues and the cost of development. Oecologia 138:350–359
Laurila A, Kujasalo J, Ranta E (1997) Different antipredator behaviour in two anuran tadpoles: effects of predator diet. Behav Ecol Sociobiol 40:329–336
Laurila A, Kujasalo J, Ranta E (1998) Predator-induced changes in life history in two anuran tadpoles: effects of predator diet. Oikos 83:307–317
Laurila A, Järvi-Laturi M, Pakkasmaa S, Merilä J (2004) Temporal variation in predation risk: stage dependency, graded responses and fitness costs in tadpole antipredator defences. Oikos 107:90–99
Laurila A, Pakkasmaa S, Merilä J (2006) Population divergence in growth rate and antipredator defences in Rana arvalis. Oecologia 147:585–595
Mathis A, Smith RJF (1993) Fathead minnows, Pimephales promelas, learn to recognize northern pike, Esox lucius, as predators on the basis of chemical stimuli from minnows in the pike’s diet. Anim Behav 46:645–656
McCollum SA, Leimberger JD (1997) Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109:615–621
McCollum SA, Van Buskirk J (1996) Costs and benefits of a predator-induced polyphenism in the gray treefrog Hyla chrysoscelis. Evolution 50:583–593
McCoy MW, Touchon JC, Landberg T, Warkentin KM, Vonesh JR (2012) Prey responses to predator chemical cues: Disentangling the importance of the number and biomass of prey consumed. PLoS One 7:e47495
Moran NA (1992) The evolutionary maintenance of alternative phenotypes. Am Nat 139:971–989
Peacor SD (2006) Behavioural response of bullfrog tadpoles to chemical cues of predation risk are affected by cue age and water source. Hydrobiologia 573:39–44
Persons MH, Walker SE, Rypstra AL, Marshall SD (2001) Wolf spider predator avoidance tactics and survival in the presence of diet-associated predator cues (Araneae: Lycosidae). Anim Behav 61:43–51
Petranka J, Hayes L (1998) Chemically mediated avoidance of a predatory odonate (Anax junius) by American toad (Bufo americanus) and wood frog (Rana sylvatica) tadpoles. Behav Ecol Sociobiol 42:263–271
Pettersson LB, Nilsson PA, Brönmark C (2000) Predator recognition and defence strategies in crucian carp, Carassius carassius. Oikos 88:200–212
Pijanowska J (1997) Alarm signals in Daphnia? Oecologia 112:12–16
Relyea RA (2003) How prey respond to combined predators: a review and an empirical test. Ecology 84:1827–1839
Relyea RA, Werner EE (2000) Morphological plasticity in four larval anurans distributed along an environmental gradient. Copeia 2000:178–190
Richardson JL (2006) Novel features of an inducible defense system in larval tree frogs (Hyla chrysoscelis). Ecology 87:780–787
Schoeppner NM, Relyea RA (2005) Damage, digestion, and defence: the roles of alarm cues and kairomones for inducing prey defences. Ecol Lett 8:505–512
Schoeppner NM, Relyea RA (2008) Detecting small environmental differences: risk-response curves for predator-induced behavior and morphology. Oecologia 154:743–754
Schoeppner NM, Relyea RA (2009) Interpreting the smells of predation: how alarm cues and kairomones induce different prey defences. Funct Ecol 23:1114–1121
Stauffer HP, Semlitsch RD (1993) Effects of visual, chemical and tactile cues of fish on the behavioral responses of tadpoles. Anim Behav 46:355–364
Takahara T, Kohmatsu Y, Maruyama A, Yamaoka R (2008) Benefit of suites of defensive behavior induced by predator chemical cues on anuran tadpoles, Hyla japonica. Behav Ecol Sociobiol 63:235–240
Teplitsky C, Laurila A (2007) Flexible defense strategies: competition modifies investment in behavioral vs. morphological defenses. Ecology 88:1641–1646
Teplitsky C, Plenet S, Joly P (2004) Hierarchical responses of tadpoles to multiple predators. Ecology 85:2888–2894
Teplitsky C, Plenet S, Léna JP, Mermet N, Malet E, Joly P (2005) Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. J Evol Biol 18:180–190
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defences. Princeton University Press, Princeton
Turner AM (1996) Freshwater snails alter habitat use in response to predation. Anim Behav 51:747–756
Van Buskirk J (2001) Specific induced responses to different predator species in anuran larvae. J Evol Biol 14:482–489
Van Buskirk J (2002) A comparative test of the adaptive plasticity hypothesis: relationships between habitat and phenotype in anuran larvae. Am Nat 160:87–102
Van Buskirk J (2009) Natural variation in morphology of larval amphibians: phenotypic plasticity in nature? Ecol Monogr 79:681–705
Van Buskirk J, Arioli M (2002) Dosage response of an induced defense: how sensitive are tadpoles to predation risk? Ecology 83:1580–1585
Van Buskirk J, McCollum SA (2000) Influence of tail shape on tadpole swimming performance. J Exp Biol 203:2149–2158
Van Buskirk J, Krugel A, Kunz J, Miss F, Stamm A (2014) The rate of degradation of chemical cues indicating predation risk: an experiment and review. Ethology 120:942–949
Vilhunen S, Hirvonen H (2003) Innate antipredator responses of Arctic charr (Salvelinus alpinus) depend on predator species and their diet. Behav Ecol Sociobiol 55:1–10
Wilson RS, Kraft PG, Van Damme R (2005) Predator-specific changes in the morphology and swimming performance of larval Rana lessonae. Funct Ecol 19:238–244
Winkler JD, Van Buskirk J (2012) Influence of experimental venue on phenotype: multiple traits reveal multiple answers. Funct Ecol 26:513–521
Wisenden BD, Chivers DP, Smith RJF (1995) Early warning in the predation sequence: a disturbance pheromone in Iowa darters (Etheostoma exile). J Chem Ecol 21:1469–1480
Wisenden BD, Chivers DP, Smith RJF (1997) Learned recognition of predation risk by Enallagma damselfly larvae (Odonata, Zygoptera) on the basis of chemical cues. J Chem Ecol 23:137–151
Acknowledgments
We thank Anssi Laurila for discussions on the experimental design. The City of Vienna (MA22-231/2011) and Land Steiermark (FA13C-53S-7/2011-92) issued permissions for collecting animals, the Ethical Commission of the University of Veterinary Medicine approved the experiments in accordance with Good Scientific Practice guidelines and national legislation. The Pilisi Parkerdő Zrt. allowed us to use their forestry roads. Research was supported by the Swiss National Science Foundation (SNF, 31003A-140979), the Lendület programme of the Hungarian Academy of Sciences (MTA, LP2012-24/2012) and an FP7 Marie Curie Career Integration Grant (PCIG13-GA-2013-631722).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Ross Andrew Alford.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Box 1: Clarifying terminology and classifying mechanisms for chemosensory-mediated predator detection
Box 1: Clarifying terminology and classifying mechanisms for chemosensory-mediated predator detection
Many studies report the use of chemical cues to detect predators, but they employ widely different definitions and classifications of types of cues. The same terms are used sometimes as synonyms, at other times they refer to different phenomena, and definitions are often missing. For example, ‘diet-released cues’ can refer to those that originate from digested prey (e.g., Ferrari et al. 2007; Ferland-Raymond et al. 2010), but sometimes they also include cues that are released by prey upon attack (e.g., Laurila et al. 1997; El-Balaa and Blouin-Demers 2013). Many authors use the term kairomone in reference to cues from a predator that are independent of its recent feeding history (e.g., Brönmark and Hansson 2000; Hettyey et al. 2010), others state that kairomones include digestion-released cues (e.g., Kats and Dill 1998; Schoeppner and Relyea 2005, 2009), and still others use the term kairomone whenever the receiver is a heterospecific (e.g., Chivers and Smith 1998).
A second example of inconsistent terminology is the classification of cues as indirect or direct. Indirect cues originate from prey and have evolved to alert other prey to predation threat. They include several kinds of chemicals: general prey metabolites that are excreted actively upon stress (‘no-cost disturbance signals’; Wisenden et al. 1995; Kiesecker et al. 1999), special disturbance cues that are costly to produce and are released by prey actively upon attack (‘alarm pheromones’; Fraker et al. 2009), cues that are passively released from injured prey tissue (‘damage-released cues’; Chivers and Smith 1998), and cues that are released from prey by digestion (‘digestion-released cues’, also referred to as ‘predator-labelling’; Mathis and Smith 1993; Chivers and Smith 1998; Ferrari et al. 2007). Direct cues, on the other hand, originate directly from the predator and represent the smell of the predator itself that is independent from its recent feeding history. These cues are released ‘unintentionally’, alerting potential prey to predation threat and lowering the predator’s chance of successful attack. Direct cues include chemicals and tissue fragments that are released more or less continually from the integument of the predator (‘kairomones’; Petranka and Hayes 1998; Brönmark and Hansson 2000), saliva released during capture and consumption of prey (we know of no study demonstrating this), and digestive body fluids of the predator, tissue fragments of the predators’ digestive tract and samples of the predators’ gut flora released during excretion (‘digestion-released cues’; Mathis and Smith 1993; Ferrari et al. 2007). As can be seen from the above list, excrements of predators may contain both indirect and direct cues. Furthermore, kairomones may be released not only continually from the integument of predators, but also during defecation (fractions of ‘digestion-released cues’). This further confuses functional and physiological/mechanistic classification. Finally, some of the current nomenclature is based on functionality and some on the timing of release, while cue origin is only implicitly understood.
To improve clarity, help avoid misunderstandings and facilitate comparability of results, we propose a new terminology for the cues involved in chemosensory-mediated predator detection. We suggest using a binomial nomenclature and classification based on the timing of cue release (stress-, attack-, capture-, digestion- or continually-released cues) in combination with cue origin (prey-borne versus predator-borne cues) (Table 1).
Rights and permissions
About this article
Cite this article
Hettyey, A., Tóth, Z., Thonhauser, K.E. et al. The relative importance of prey-borne and predator-borne chemical cues for inducible antipredator responses in tadpoles. Oecologia 179, 699–710 (2015). https://doi.org/10.1007/s00442-015-3382-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3382-7