Abstract
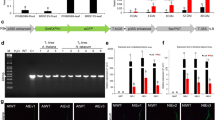
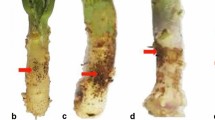
Take-all (caused by the fungal pathogen Gaeumannomyces graminis var. tritici, Ggt) and common root rot (caused by Bipolaris sorokiniana) are devastating root diseases of wheat (Triticum aestivum L.). Development of resistant wheat cultivars has been a challenge since no resistant wheat accession is available. GmPGIP3, one member of polygalacturonase-inhibiting protein (PGIP) family in soybean (Glycine max), exhibited inhibition activity against fungal endopolygalacturonases (PGs) in vitro. In this study, the GmPGIP3 transgenic wheat plants were generated and used to assess the effectiveness of GmPGIP3 in protecting wheat from the infection of Ggt and B. sorokiniana. Four independent transgenic lines were identified by genomic PCR, Southern blot, and reverse transcription PCR (RT-PCR). The introduced GmPGIP3 was integrated into the genomes of these transgenic lines and could be expressed. The expressing GmPGIP3 protein in these transgenic wheat lines could inhibit the PGs produced by Ggt and B. sorokiniana. The disease response assessments postinoculation showed that the GmPGIP3-expressing transgenic wheat lines displayed significantly enhanced resistance to both take-all and common root rot diseases caused by the infection of Ggt and B. sorokiniana. These data suggested that GmPGIP3 is an attractive gene resource in improving resistance to both take-all and common root rot diseases in wheat.




Similar content being viewed by others
References
Aguero CB, Uratsu SL, Greve C, Powell AT, Labavitch JM, Meredith CP, Dandekar AM (2005) Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol 6:43–51
Bithell SL, Butler RC, Harrow S, McKay A, Cromey MG (2011) Susceptibility to take-all of cereal and grass species, and their effects on pathogen inoculum. Ann Appl Biol 159:252–266
Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL (2008) Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci 13:610–617
Cervone F, Castoria R, Leckie F, De Lorenzo G (1997) Perception of fungal elicitors and signal transduction. In: Aducci P (ed) Signal Transduction in plants. Birkhauser Verlag, Basel
Chen L, Zhang ZY, Liang HX, Liu HX, Du LP, Xu HJ, Xin ZY (2008) Overexpression of TiERF1 enhances resistance to sharp eyespot in transgenic wheat. J Exp Bot 59:4195–4204
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5:213–218
Clay RP, Bergmann CW, Fuller MS (1997) Isolation and characterization of an endopolygalacturonase from Cochliobolus sativus and a cytological study of fungal penetration of barley. Phytopathology 87:1148–1159
D’Ovidio R, Mattei B, Roberti S, Bellincampi D (2004a) Polygalacturonase, poly- galacturonase-inhibiting proteins and pectic oligomers in plant-pathogen interactions. Biochim Biophys Acta 1696:237–244
D’Ovidio R, Raiola A, Capodicasa C, Devoto A, Pontiggia D, Roberti S, Galletti R, Conti E, O’Sullivan D, De Lorenzo G (2004b) Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins (PGIPs) reveals sub-functionalization for defense against fungi and insects. Plant Physiol 135:2424–2435
D’Ovidio R, Roberti S, Di Giovanni M, Capodicasa C, Melaragni M, Sella L, Tosi P, Favaron F (2006) The characterization of the soybean polygalacturonase-inhibiting proteins (pgip) gene family reveals that a single member is responsible for the activity detected in soybean tissues. Planta 224:633–645
Daval S, Lebreton L, Gazengel K, Boutin M, Guillerm-Erckelboudt AY, Sarniguet A (2011) The biocontrol bacterium Pseudomonas fluorescens Pf29Arp strain affects the pathogenesis-related gene expression of the take-all fungus Gaeumannomyces graminis var. tritici on wheat roots. Mol Plant Pathol 12:839–854
De Lorenzo G, D’Ovidio R, Cervone F (2001) The role of polygalacturonase- inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu Rev Phytopathol 39:313–335
Dong N, Liu X, Lu Y, Du LP, Xu HJ, Liu HX, Xin ZY, Zhang ZY (2010) Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct Integr Genomics 10:215–226
Ferrari S, Vairo D, Ausubel FM, Cervone F, de Lorenzo G (2003) Tandemly duplicated Arabidopsis genes that encode polygalacturonase- inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15:93–106
Ferrari S, Sella L, Janni M, De Lorenzo G, Favaron F, D’Ovidio R (2012) Transgenic expression of polygalacturonase-inhibiting proteins in Arabidopsis and wheat increases resistance to the flower pathogen Fusarium graminearum. Plant Biol 14:31–38
Fu D, Uauy C, Distelfeld A, Blechl A, Epstein L, Chen X, Sela H, Fahima T, Dubcovsky J (2009) A kinase-START gene confers temperature dependent resistance to wheat stripe rust. Science 323:1357–1360
Gutteridge RJ, Bateman GL, Todd AD (2003) Variation in the effects of take-all disease on grain yield and quality of winter cereals in field experiments. Pest Manag Sci 59:215–224
Huang Q, Allen C (2000) Polygalacturonases are required for rapid colonization and full virulence of Ralstonia solanacearum on tomato plants. Physiol Mol Plant Pathol 57:176–186
Isshiki A, Akimitsu K, Yamamoto M, Yamamoto H (2001) Endopolygalacturonase is essential for citrus black rot caused by Alternaria citri but not brown spot caused by Alternaria alternata. Mol Plant Microbe Interact 14:749–757
Janni M, Sella L, Favaron F, Blechl AE, De Lorenzo G, D’Ovidio R (2008) The expression of a bean PGIP in transgenic wheat confers increased resistance to the fungal pathogen Bipolaris sorokiniana. Mol Plant Microbe Interact 21:171–177
Janni M, Bozzini T, Moscetti I, Volpi C, D’Ovidio R (2013) Functional characterisation of wheat Pgip genes reveals their involvement in the local response to wounding. Plant Biol (Stuttg) 15:1019–1024
Jones DA, Jones JDG (1997) The roles of leucine rich repeats in plant defences. Adv Bot Res 24:90–167
Joubert DA, Slaughter AR, Kemp G, Becker VWJ, Krooshof GH, Bergmann C, Benen J, Pretorius IS, Vivier MA (2006) The grapevine polygalacturonase-inhibiting protein (VvPGIP1) reduces Botrytis cinerea susceptibility in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Res 15:687–702
Kumar J, Schäfer P, Hückelhoven R, Langen G, Baltruschat H, Stein E, Nagarajan S, Kogel K (2002) Bipolaris sorokiniana, a cereal pathogen of global concern: cytological and molecular approaches towards better control. Mol Plant Pathol 3:185–195
Laluk K, Mengiste T (2010) Necrotroph attacks on plants: wanton destruction or covert extortion? Arabidopsis Book 8:e0136
Li R, Rimmer R, Yu M, Sharpe AG, Seguin-Swartz G, Lydiate D, Hegedus DD (2003) Two Brassica napus polygalacturonase inhibitory protein genes are expressed at different levels in response to biotic and abiotic stresses. Planta 217:299–308
Liu X, Yang L, Zhou X, Zhou M, Lu Y, Ma L, Ma H, Zhang Z (2013) Transgenic wheat expressing Thinopyrum intermedium MYB transcription factor TiMYB2R-1 shows enhanced resistance to the take-all disease. J Exp Bot 64:2243–2253
Lu L, Zhou F, Zhou Y, Fan X, Ye S, Wang L, Chen H, Lin Y (2012) Expression profile analysis of the polygalacturonase-inhibiting protein genes in rice and their responses to phytohormones and fungal infection. Plant Cell Rep 31:1173–1187
Oeser B, Heidrich PM, Muller U, Tudzynski P, Tenberge KB (2002) Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet Biol 36:176–186
Powell AL, Van Kan J, Ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM (2000) Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol Plant Microbe Interact 13:942–950
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967
Rodriguez-Palenzuela P, Burr TJ, Collmer A (1991) Polygalacturonase is a virulence factor in Agrobacterium tumefaciens biovar 3. J Bacteriol 173:6547–6552
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A 81:8014–8019
Sharp PJ, Chao S, Desai S, Gale MD (1989) The isolation, characterization and application in the Triticeae of a set of wheat RFLP probes identifying each homoeologous chromosome arm. Theor Appl Genet 78:342–348
Shivanna MB, Meera MS, Hyakumachi M (1996) Role of root colonization ability of plant growth promoting fungi in the suppression of take-all and common root rot of wheat. Crop Prot 15:497–504
Taylor J, Secor A (1988) An improved diffusion assay for quantifying the polygalacturonase content of Ervinia culture filtrates. Phytopathology 78:1101–1103
ten Have A, Mulder W, Visser J, van Kaan JAL (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant-Microbe Interact 11:1009–1016
Wang X, Zhu X, Tooley P, Zhang X (2013) Cloning and functional analysis of three genes encoding polygalacturonase-inhibiting proteins from Capsicum annuum and transgenic CaPGIP1 in tobacco in relation to increased resistance to two fungal pathogens. Plant Mol Biol 81:379–400
Xu HJ, Pang JL, Ye XG, Du LP, Li LC, Xin ZY, Ma YZ, Chen JP, Chen J, Chen SH, Wu HY (2001) Study on the gene transferring of Nib8 into wheat for its resistance to the yellow mosaic virus by bombardment. Acta Agron Sin 27:684–689 (in Chinese with English abstract)
Zhang Z, Liu X, Wang X, Zhou M, Zhou X, Ye X, Wei X (2012) An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defense- and stress- related genes. New Phytol 196:1155–1170
Zuppini A, Navazio L, Sella L, Castiglioni C, Favaron F, Mariani P (2005) An endopolygalacturonase from Sclerotinia sclerotiorum induces calcium-mediated signaling and programmed cell death in soybean cells. Mol Plant Microbe Interact 18:849–855
Acknowledgments
This study was supported by the National “Key Sci-Tech” program, China (Grant no.2013ZX08002001-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Aiyun Wang and Xuening Wei contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, A., Wei, X., Rong, W. et al. GmPGIP3 enhanced resistance to both take-all and common root rot diseases in transgenic wheat. Funct Integr Genomics 15, 375–381 (2015). https://doi.org/10.1007/s10142-014-0428-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-014-0428-6