Abstract
Main conclusion
Screening for resistance in 40 potato genotypes to Rhizoctonia solani AG-3PT-stem-canker, antioxidant enzymes activity as well as total phenol compounds were documented.
Abstract
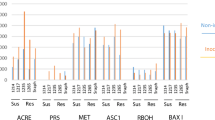
Rhizoctonia solani AG-3PT-stem-canker is one of the most devastating diseases that leads to severe economic losses in potatoes, Solanum tuberosum globally. Crop management and eugenic practices, especially the use of resistance can be effective in reducing the disease incidence. However, the information about potato-R. Solani interaction is still limited. This study explored screening for resistance in forty potato genotypes to R. solani, analyzing biomass growth parameters (BGPs), as well as antioxidant enzymes activity of which peroxidase/peroxide-reductases (POXs), superoxide dismutase (SOD), polyphenol oxidase (PPO), catalase (CAT), phenylalanine ammonia-lyase (PAL), β-1,3-glucanase (GLU) and total phenol compounds (TPCs) were taken into account. In addition, we analyzed up-regulation of two gene markers (PR-1 and Osmotin), using reverse transcription quantitative PCR (RT-qPCR). For which, the resistant ‘Savalan’, partially resistant ‘Agria’, partially susceptible ‘Sagita’ and susceptible ‘Pashandi’ were selected to explore the trails in their roots and leaves over the time courses of 1, 2 and 3-weeks post inoculation (wpi) following inoculation. Cluster analysis divided potatoes into four distinct groups, based on disease severity scales (0–100%) significance. The BGPs, shoot and root length, fresh and dry weight, and root volume were also significantly higher in infected potatoes compared to non-inoculated controls. Antioxidant enzymes activity also indicated the highest increased levels for POX (fourfold at 3wpi), CAT (1.5-fold at 3wpi), SOD (6.8-fold at 1wpi), and PAL (2.7-fold at 3wpi) in the resistant genotype, ‘Savalan’, whereas the highest activity was recorded in TPC (twofold at 1 wpi), PPO (threefold at 3wpi), and GLU (2.3-fold at 1wpi) in partially resistant genotypes. Although the defense-related enzymatic activities were sharply elevated in the resistant and partially resistant genotypes following inoculation, no significant correlations were between the activity trends of the related enzymes. The two related gene markers also showed comprehensive transcriptional responses up to 3.4-fold, predominantly in resistant genotypes. Surprisingly, the PR-1 gene marker, basically resistant to Wilting agent Verticillium dahlia was overexpressed in resistant 'Savalan' and 'Agria' against R. solani AG3-PT. Similar results were obtained on Osmotin gene marker resistant to late-blight P. infestans, and early-blight Alternaria solani that similarly modulates immunity against R. solani. Furthermore, there was a significant correlation between resistance, enzyme activity, and gene expression in the aforesaid cultivars. Studying the physiological metabolic pathways of antioxidant enzymes activity appears to be an important direction in research to elucidate resistance to R. solani in potatoes.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Abbreviations
- AG:
-
Anastomosis group
- BGPs:
-
Biomass growth parameters
- CAT:
-
Catalase
- GLU:
-
β-1,3-Glucanase
- PAL:
-
Phenylalanine ammonia-lyase
- PPO:
-
Polyphenol-oxidase
- POX:
-
Peroxidase/peroxide-reductases
- SOD:
-
Superoxide dismutase
- TPC:
-
Total phenol compounds
- wpi:
-
Weeks post-inoculation
References
Abdelrahman M, Al-Sadi AM, Pour-Aboughadarehd AR, BurritteLam-Son DJ (2019) Genome editing using CRISPR/Cas9–targeted mutagenesis: an opportunity for yield improvements of crop plants grown under environmental stresses. Plant Physiol Biochem 131:31–36. https://doi.org/10.1016/j.plaphy.2018.03.012
Ali S, Mir ZA, Tyagi A, Mehari H, Meena RP, Bhat JA, Yadav P, Papalou P, Rawat S, Grover A (2017) Overexpression of NPR1 in Brassica juncea confers broad spectrum resistance to fungal pathogens. Front Plant Sci 8:1693. https://doi.org/10.3389/fpls.2017.01693
Alonso MD, Lomako J, Lomako WM, Whelan WJ (1995) A new look at the biogenesis of glycogen. FASEB J 9:1126–1137
Bach CE, Warnock DD, Van Horn DJ, Weintraub MN, Sinsabaugh RL, Allison SD, German DP (2013) Measuring phenol oxidase and peroxidase activities with pyrogallol, L-DOPA, and ABTS: effect of assay conditions and soil type. Soil Biol Biochem 67:183–191. https://doi.org/10.1016/j.soilbio.2013.08.022
Bagheri LM, Nasr-Esfahani M, Abdossi V, Naderi D (2020) Analysis of candidate genes expression associated with defense responses to root and collar rot disease caused by Phytophthora capsici in peppers Capsicum annuum. Genomics 112:2309–2317. https://doi.org/10.1016/j.ygeno.2020.01.002
Bari R, Jones JDG (2009) Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488. https://doi.org/10.1007/s11103-008-9435-0
Bolwell GP, Daudi A (2009) Reactive oxygen species in plant–pathogen interactions. In: Rio L, Puppo A (Eds) Reactive oxygen species in plant signaling. Signaling and communication in plants. Springer, Berlin, Heidelberg, pp 113–131. https://doi.org/10.1007/978-3-642-00390-5-7
Boyd LA, Ridout C, O’Sullivan DM, Leach JE, Leung H (2013) Plant–pathogen interactions: disease resistance in modern agriculture. Trends Genet 29:233–240. https://doi.org/10.1016/j.tig.2012.10.011
Claiborne A (2018) Catalase activity. CRC handbook of methods for oxygen radical research. CRC Press, pp 283–284
Cordovez V, Mommer L, Moisan K, Lucas-Barbosa D, Pierik R, Mumm R, Carrion VJ, Raaijmakers JM (2017) Plant phenotypic and transcriptional changes induced by volatiles from the fungal root pathogen Rhizoctonia solani. Plant Sci 8:1–14. https://doi.org/10.3389/fpls.2017.01262
Derksen H, Badawi M, Henriquez MA, Yao Z, El-Bebany AF, Daayf F (2013) Differential expression of potato defence genes associated with the salicylic acid defense signaling pathway in response to weakly and highly aggressive isolates of Verticillium dahliae. J Phytopath 161: 142–53. 10.1111 /jph.12038
Dievart A, Gottin C, Périn C, Ranwez V, Chantret N (2020) Origin and diversity of plant receptor-like kinases. Annu Rev Plant Biol 71:131–156. https://doi.org/10.1146/annurev-arplant-073019-025927
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Ferrucho RL, Cifuentes JM, Ceresini P, García-Domínguez C (2012) Rhizoctonia solani AG-3PT is the major pathogen associated with potato stem canker and black scurf in Colombia. Agron Colomb 30:204–213
Fiers M, Edel-Hermann V, Heraud C, Gautheron N, Chatot C, Hingrat YL, Bouchek-Mechiche K, Steinberg C (2011) Genetic diversity of Rhizoctonia solani associated with potato tubers in France. Mycologia 103:1230–1244. https://doi.org/10.3852/10-231
Forghani D, Bazgir E, Nasr-Esfahani M, Darvishnia M (2021) Genomic structure of novel Iranian Rhizoctonia solani AG-3PT isolates on potato, Solanum tuberosum. Sydowia 73: 217–238. 10.12905 /0380
Gallou A, Cranenbrouck S, Declerck S (2009) Trichoderma harzianum elicits defence response genes in roots of potato plantlets challenged by Rhizoctonia solani. Eur J Plant Pathol 124:219–230. https://doi.org/10.1007/s10658-008-9407-x
Gangadhar BH, Mishra RK, Kappachery S, Baskar V, Venkatesh J, Nookaraju A, Thiruvengadam M (2021) Enhanced thermo-tolerance in transgenic potato (Solanum tuberosum L.) overexpressing hydrogen peroxide-producing germin-like protein (GLP). Genomics 113:3224–3234. https://doi.org/10.1016/j.ygeno.2021.07.013
Ghasemi AR, Golparvar AR, Isfahani MN (2014) Analysis of genetic diversity of sugar beet genotypes using random amplified polymorphic DNA marker. Genetika 46:975–984. https://doi.org/10.2298/GENSR1403975G
Gholamaliyan A, Nasr-Esfahani M, Dababat AA ( 2021) Novel Iranian wheat cultivars resistant to Bipolaris sorokiniana. Sydowia 73: 257–269. https://doi.org/10.12905/0380.sydowia73-2021-0217
Gururani MA, Venkatesh J, Upadhyaya CP, Nookaraju A, Pandey SK, Park SW (2012) Plant disease resistance genes: current status and future directions. Physiol Mol Plant Pathol 78:51–65. https://doi.org/10.1016/j.pmpp.2012.01.002
Gvozdeva EL, Volotskaya AV, Sofin AV, Kudryavtseva NN (2006) Interaction of proteinases secreted by the fungal plant pathogen Rhizoctonia solani with natural proteinase inhibitors produced by plants. Appl Biochem Microbiol 42:502–507. https://doi.org/10.1134/S0003683806050103
Hakim Ullah A, Hussain A, Shaban M, Khan AH, Alariqi M et al (2018) Osmotin: A plant defense tool against biotic and abiotic. Plant Physiol Biochem 123:149–159. https://doi.org/10.1016/j.plaphy.2017.12.012
Hashemi L, Golparvar AR, Nasr-Esfahani M, Golabadi M (2019) Correlation between cucumber genotype and resistance to damping-off disease caused by Phytophthora melonis. Biotechnol Equip 33:1494–1504. https://doi.org/10.1007/s11033-020-05520-5
Hashemi L, Golparvar AR, Nasr-Esfahani M, Golabadi M (2020) Regulation of novel candidate genes resistant in response to Phytophthora melonis in cucumber Cucumis sativus. Mol Biol Rep 142:500–509. https://doi.org/10.1007/s11033-020-05520-5
Hong ZH, Fen XU, Yu WU, Hong-hai HU, Xiao-feng DAI (2017) Progress of potato staple food research and industry development in China. J Integr Agric 16:2924–2932. https://doi.org/10.1016/S2095-3119(17)61736-2
Hyun MW, Yun YH, Kim JY, Kim SH (2011) Fungal and plant phenylalanine ammonia-lyase. Mycobiology 39:257–265. https://doi.org/10.5941/MYCO.2011.39.4.257
Jiang S, Han S, He D, Cao G, Fang K, Xiao X, Yi J (2019) The accumulation of phenolic compounds and increased activities of related enzymes contribute to early defense against walnut blight. Physiol Mol Plant Pathol 108:101433. https://doi.org/10.1016/j.pmpp.2019.101433
Kapoor D, Singh S, Kumar V, Romero R, Prasad R, Singh J (2019) Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 19:100182. https://doi.org/10.1016/j.plgene.2019.100182
Kaur A, Sudhakara Reddy M, Pati PK, Kaur A (2020) Over-expression of Osmotin (OsmWS) gene of Withania somnifera in potato cultivar ‘Kufri Chipsona 1’ imparts resistance to Alternaria solani. Plant Cell Tiss Organ Cult 142:131–142. https://doi.org/10.1007/s11240-020-01847-w
Kettles GJ, Luna E (2019) Food security in 2014: How do we control the fungal threat? Fungal Biol 123:558–564. https://doi.org/10.1016/j.funbio.2019.04.006
Khan A, Li RJ, Sun JT, Ma F, Zhang HX, Jin J (2018) Genome-wide analysis of dirigent gene family in pepper (Capsicum annuum L.) and characterization of CaDIR7 in biotic and abiotic stresses Sci Rep 8: 5500. https://doi.org/10.1038/s41598-018-23761-0
Khatediya NK, Parmar DV, Mahatma MK (2018) Increased accumulation of phenolic metabolites in groundnut (Arachis hypogaea L.) genotypes contribute to defense against Sclerotium rolfsii infection. Arch Phytopathol Plant Protec 51:530–549. https://doi.org/10.1080/03235408.2018.1490519
Kim DS, Hwang BK (2014) An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid dependent signalling of the defense response to microbial pathogens. J Exp Bot 65:2295–2306. https://doi.org/10.1093/jxb/eru109
Kim N, Kang WH, Lee J, Yeom SI (2019) Development of clustered resistance gene analogs-based markers of resistance to Phytophthora capsici in chili pepper. Biol Med Res Int. https://doi.org/10.1155/2019/1093186
Li Y, Lopez P, Durand P, Ouazzani J, Badet B, Badet-Denisot MA (2007) An enzyme-coupled assay for amidotransferase activity of glucosamine-6-phosphate synthase. Anal Biochem 370:142–146. https://doi.org/10.1016/j.ab.2007.07.031
Li J, Mao L, Zhang Y, Zhang L, Jiang H (2018) Phytochemical changes in mango fruit in response to Alternaria alternata infection. Czech J Food Sci 36: 227–232. 10.17221/3 28/2017-CJFS
Liu D, Raghothama KG, Hasegawa PM, Bressan RA (1994) Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci USA 91:1888–1892. https://doi.org/10.1073/pnas.91.5.1888
Marcou S, Wikström M, Ragnarsson S, Persson L, Höfte M (2021) Occurrence and anastomosis grouping of Rhizoctonia spp. inducing black scurf and greyish-white felt-like mycelium on carrot in Sweden. J Fungi 7: 396. https://doi.org/10.3390/jof7050396
Matern U, Kneusal RE (1998) Phenolic compounds in plant disease resistance. Phytoparasitica 16:153–170
Mathur M, Naira A, Kadooa A (2020) Plant-pathogen interactions: microRNA-mediated trans-kingdom gene regulation in fungi and their host plants. Genomics 112:3021–3035. https://doi.org/10.1016/j.ygeno.2020.05.021
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–429. https://doi.org/10.1021/ac60147a030
Moatamedi M, Bazgir E, Nasr-Esfahani M, Darvishnia M (2018) Genetic variation of bread wheat accessions in response to cereal cyst nematode, Heterodera filipjevi. Nematodology 20: 859–875. https://doi.org/10.1163/15685411-00003181
Moghaddam GA, Rezayatmand Z, Nasr-Esfahani M, Khozae M (2019) Genetic defense analysis of tomatoes in response to early blight disease, Alternaria alternata. Plant Physiol Biochem 142:500–509. https://doi.org/10.1016/j.plaphy.2019.08.011
Moghaddam GA, Rezayatmand Z, Nasr-Esfahani M, Khozaei M (2020) Bio-genetic analysis of resistance in tomato to early blight disease, Alternaria alternata. Phytochemistry 179: 112486. https://doi.org/10.1016/j.phytochem.2020.112486
Moghaddam GA, Nasr-Esfahani M, Rezayatmand Z, Khozaei M (2022) Genomic markers analysis associated with resistance to Alternaria alternata (fr.) keissler—tomato pathotype, Solanum lycopericum L. Breeding Sci 72:285–296. https://doi.org/10.1270/jsbbs.22003
Mohammadbagheri LM, Nasr-Esfahani M, Al-Sadi AM, Hassanzadeh Khankahdani H, Ghadirzadeh E (2022) Screening for resistance and genetic population structure associated with Phytophthora capsici-pepper root and crown rot. Physiol Mol Plant Pathol 119:101835
Mohammadbagheri L, Nasr-Esfahani M, Abdossi V, Naderi D (2021) Genetic diversity and biochemical analysis of Capsicum annuum (bell pepper) in response to root and basal rot disease, Phytophthora capsici. Phytochemistry 190: 112884. https://doi.org/10.1016/j.phytochem.2021.112884
Monazzah M, Tahmasebi-Enferadi S, Rabiei Z (2018) Enzymatic activities and pathogenesis-related genes expression in sunflower inbred lines affected by Sclerotinia sclerotiorum culture filtrate. J Appl Microbiol 125:227–242
Monazzah M, Nasr-Esfahani M, Tahmasebi S (2022) Genetic structure and proteomic analysis associated in potato to Rhizoctonia solani AG-3PT stem canker and black scurf. Physiol Mol Plant Pathol 112:101905. https://doi.org/10.1016/j.pmpp.2022.101905
Naderi N, Nasr-Esfahani M, Bakhshi Khaniki G (2020) Analysis of molecular characterizations of beets, Beta vulgaris in response to cyst nematodes, Heterodera schachtii. Physiol Mol Plant Pathol 112: 101518. https://doi.org/10.1016/j.pmpp.2020.101518.
Nasehi A, Al-Sadi AM, Nasr-Esfahani M, Alsultan W, Javan-Nikkhah M (2019) Molecular re-identification of Stemphylium lycopersici and Stemphylium solani isolates deposited in NCBI GenBank and morphological characteristics of Malaysian isolates. Eur J Plant Pathol 153:965–974. https://doi.org/10.1007/s10658-018-1602-9
Nasr-Esfahani M (2005) Susceptibility assessment of potato cultivars to Fusarium dry rot species. Potato Res 48:215–226. https://doi.org/10.1007/BF02742378
Nasr Esfahani M, Ansari Pour B (2008) Differences in resistance in onion cultivars to pink root rot disease in Iran. J Gen Plant Pathol 74:235–244. https://doi.org/10.1007/s10327-007-0070-4
Nasr-Esfahani M, Chatraee M, Shafizadeh S (2012) Evaluation of resistance of cucurbit and cucumber cultivars to Phytophthora drechsleri in greenhouse. Iranian Seed Plant Improvement J 28:407–417
Nasr-Esfahani M, Nasehi A, Rahmanshirazi P, Ghadirian H, Ashtiani FA (2014) Susceptibility assessment of bell pepper genotypes to crown and root rot disease. Arch Phytopathol Plant Protect 47: 944–953. https://doi.org/10.24425/jppr.2020.132201
Nasr-Esfahani M, Hashemi L, Nasehi A, Nasr-Esfahani A, Nasr-Esfahani A (2020) Novel Cucumis enzymes associated with host-specific disease resistance to Phytophthora melonis Katsura. Biotechnol Equip 34:873–884. https://doi.org/10.1080/13102818.2020.1810123
Nasr-Esfahani M (2020) Genetic variability and virulence of some Iranian Rhizoctonia solani isolates associated with stem canker and black scurf of potato (Solanum tuberosum L.). J Plant Protec Res 60:21–30. https://doi.org/10.24425/jppr.2020.132201
Nicot N, Hausman JF, Hoffmann L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914. https://doi.org/10.1093/jxb/eri285
Paranidharan V, Palaniswami A, Vidhyasekaran P, Velazhahan R (2003) Induction of enzymatic scavengers of active oxygen species in rice in response to infection by Rhizoctonia solani. Acta Physiol Plant 25: 91–96. https://doi.org/10.1007/s11738-003-0041-0
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29: e45–e45. https://doi.org/10.1093/nar/29.9.e45
Qalavand F, Nasr-Esfahan M, Vatandoost J, Azarm DA (2022a) Enzyme activity and population genetic structure analysis in wheat associated with resistance to Bipolaris sorokiniana-common root rot diseases. Phytochemistry 200:113208. https://doi.org/10.1016/j.phytochem.2022.113208
Qalavand F, Nasr-Esfahan M, Vatandoost J, Azarm DA (2022b) Transcriptome - based analysis of resistance mechanism to Bipolaris sorokiniana - wheat common root rot disease. Plant Biol (Stuttg https://doi.org/10.1111/plb.13470
Rychlik W (2007) OLIGO 7 primer analysis software. Methods Mol Biol 402:35–60
Sadeghpour N, Asad-Gharne HA, Nasr-Esfahani M, Khankahdani H, Golabadi M (2022) Antioxidant enzymes associated with resistance to Fusarium oxysporum f. sp. melonis race 1. 2 in melon. Physiol Mol Plant Pathol 126: 101880. https://doi.org/10.1016/j.pmpp.2022.101880
Saikia R, Singh BP, Kumar R, Arora DK (2005) Detection of pathogenesis-related proteins–chitinase and β-1,3-glucanase in induced chickpea. Curr Sci 89: 659–663. https://www.jstor.org/stable/24111163
Sarkar J, Chakraborty U, Chakraborty B (2018) Induced defense response in wheat plants against Bipolaris sorokiniana following application of Bacillus safensis and Ochrobactrum pseudogrignonense. Indian Phytopathol 71:49–58. https://doi.org/10.3389/fpls.2017.01806
Schröder M, Hahlbrock K, Kombrink E (1992) Temporal and spatial patterns of 1, 3-β-glucanase and chitinase induction in potato leaves infected by Phytophthora infestans. Plant J 2:161–172. https://doi.org/10.1111/j.1365-313X.1992.00161.x
Shan J, Song W, Zhou J, Wang X, Xie C, Gao X, et al. (2013) Transcriptome analysis reveals novel genes potentially involved in photoperiodic tuberization in potato. Genomics 102: 388–396. 10.1016 /j.ygeno.2013.07.001
Sousa RM, Cunha AC, Fernandes-Ferreira M (2021) The potential of Apiaceae species as sources of singular phytochemicals and plant-based pesticides. Phytochemistry 187:112714. https://doi.org/10.1016/j.phytochem.2021.112714
Sudisha J, Sharathchandra RG, Amruthesh KN, Kumar A, Shetty HS (2012) Pathogenesis related proteins in plant defense response. In: Merillon JM, Ramawat KG (Eds) Plant defence: Biological control. Springer, Dordrecht, The Netherlands, pp 379–403. https://doi.org/10.13140/2.1.2445.2163
Tehrani MM, Nasr-Esfahani M, Mousavi A, Mortezaiinezhad F, Azimi MH (2020) Regulation of related genes promoting resistant in Iris against root rot disease, Fusarium oxysporum f. sp. gladioli. Genomics 112: 3013–3020. 10.1016 /j.jplph.2009.08.003
Vera-Estrella R, Barkla BJ, Higgins VJ, Blumwald E (1994) Plant defense response to fungal pathogens (activation of host-plasma membrane H+-ATPase by elicitor-induced enzyme dephosphorylating). Plant Physiol 104:209–215. https://doi.org/10.1104/pp.104.1.209
Vidhyasekaran P (2004) Concise encyclopedia of plant pathology. CRC Press, Boca Raton, FL, USA
Vitti A, Nuzzaci M, Scopa A, Tataranni G, Remans T, Vangronsveld J, Sofo A (2013) Auxin and cytokinin metabolism and root morphological modifications in Arabidopsis thaliana seedlings infected with Cucumber mosaic virus (CMV) or exposed to cadmium. Int J Mol Sci 14:6889–6902. https://doi.org/10.3390/ijms14046889
Weinhold AR, Bowman T, Hall DH (1982) Rhizoctonia disease of potato: effect on yield and control by seed tuber treatment. Plant Dis 66:815–818
Wilson PS, Ahvenniemi PM, Lehtonen MJ, Kukkonen M, Rita H, Valkonen JPT (2008) Biological and chemical control and their combined use to control different stages of the Rhizoctonia disease complex on potato through the growing season. Ann Appl Biol 153:307–320. https://doi.org/10.1111/j.1744-7348.2008.00292.x
Yang G, Li C (2012) General description of Rhizoctonia species complex. In: Cumagun CJR (Ed) Plant Pathology. InTech Open, pp 41–52. https://doi.org/10.5772/39026
Yang X, Hu H, Yu D, Sun Z, He X, Zhang J, Chen Q (2017) Candidate resistant genes of sand pear (Pyrus pyrifolia Nakai) to Alternaria alternata revealed by transcriptome sequencing. PLoS One 10: e0135046. https://doi.org/10.1371/journal.pone.0135046
Yusuf CYL, Abdullah JO, Shaharuddin NA, Abu Seman I, Abdullah MD (2018) Characterization of promoter of EgPAL1, a novel PAL gene from the oil palm Elaeis guineensis Jacq. Plant Cell Rep 37:265–278. https://doi.org/10.1007/s00299-017-2228-7
Zhang X, Li D, Huo H, Xing X, Lian Y, Yu Z, Hao J (2021) Improving evaluation of potato resistance to Rhizoctonia solani infection by optimizing inoculum-based method combined with toxin-based assay. Crop Prot 144:105544. https://doi.org/10.1016/j.cropro.2021.105544
Acknowledgements
We thank Plant Pathology Department, Faculty of Agricultural Sciences, University of Guilan, Guilan, Iran; Plant Protection Research Department, Isfahan Center for Agricultural and Natural Resources Research and Education, AREEO, Isfahan, Iran; Plant Protection Research Department, Ardabil Center for Agricultural and Natural Resources Research and Education, AREEO, Ardabil, Iran; for providing facilities to run the project.
Funding
There was no funding source supporting this project.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Soheili-Moghaddam, B., Nasr-Esfahani, M., Mousanejad, S. et al. Biochemical defense mechanism associated with host-specific disease resistance pathways against Rhizoctonia solani AG3-PT potatoes canker disease. Planta 257, 13 (2023). https://doi.org/10.1007/s00425-022-04039-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-04039-2