Abstract
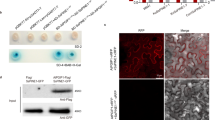
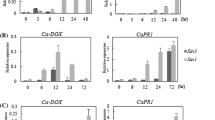
Polygalacturonase-inhibiting proteins (PGIPs) selectively inhibit polygalacturonases (PGs) secreted by invading plant pathogenic fungi. PGIPs display differential inhibition towards PGs from different fungi, also towards different isoforms of PGs originating from a specific pathogen. Recently, a PGIP-encoding gene from Vitis vinifera (Vvpgip1) was isolated and characterised. PGIP purified from grapevine was shown to inhibit crude polygalacturonase extracts from Botrytis cinerea, but this inhibitory activity has not yet been linked conclusively to the activity of the Vvpgip1 gene product. Here we use a transgenic over-expression approach to show that the PGIP encoded by the Vvpgip1 gene is active against PGs of B. cinerea and that over-expression of this gene in transgenic tobacco confers a reduced susceptibility to infection by this pathogen. A calculated reduction in disease susceptibility of 47–69% was observed for a homogeneous group of transgenic lines that was statistically clearly separated from untransformed control plants following infection with Botrytis over a 15-day-period. VvPGIP1 was subsequently purified from transgenic tobacco and used to study the specific inhibition profile of individual PGs from Botrytis and Aspergillus. The heterologously expressed and purified VvPGIP1 selectively inhibited PGs from both A. niger and B.␣cinerea, including BcPG1, a PG from B. cinerea that has previously been shown to be essential for virulence and symptom development. Altogether our data confirm the antifungal nature of the VvPGIP1, and the in vitro inhibition data suggest at least in part, that the VvPGIP1 contributed to the observed reduction in disease symptoms by inhibiting the macerating action of certain Botrytis PGs in planta. The ability to correlate inhibition profiles to individual PGs provides a more comprehensive analysis of PGIPs as antifungal genes with biotechnological potential, and adds to our understanding of the importance of PGIP:PG interactions during disease and symptom development in plants.
Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Carstens M, Vivier MA, Pretorius IS (2003) The Saccharomyces cerevisiae chitinase, encoded by the CTS1-2 gene, confers antifungal activity against Botrytis cinerea to transgenic tobacco. Transgenic Res 12:497–508
Davies C, Robinson SP (2000) Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of␣cDNAs encoding putative cell wall and stress response proteins. Plant Physiol 122:803–812
De Ascensao A (2001) Isolation and characterization of a polygalacturonase-inhibiting protein (PGIP) and its encoding gene from Vitis vinifera L. Ph.D. Thesis, Stellenbosch University, Stellenbosch, RSA
De Lorenzo G, Ferrari S (2002) Polygalacturonase-inhibiting proteins in defence against phytopathogenic fungi. Curr Opin Plant Biol 5:295–299
De Lorenzo G, Cervone F, Bellincampi D, Caprari C, Clark AJ, Desiderio A, Devoto A, Forrest R, Leckie F, Nuss L (1994) Polygalacturonase, PGIP and oligogalacturonides in cell–cell communication. Biochem Soc Trans 22:394–397
De Lorenzo G, D’Ovidio R, Cervone F (2001) The role of polygalacturonase-inhibiting proteins (PGIPs) in defence against pathogenic fungi. Annu Rev Phytopathol 39:313–335
Esquerré-Tugayé MT, Boudart G, Dumas B (1999) Cell wall-degrading enzymes, inhibitory proteins and oligosaccharides participate in the molecular dialogue between plants and pathogens. Plant Physiol Biochem 38:157–163
Favaron F, D’Ovidio RD, Porceddu E, Alghisi P (1994) Purification and molecular characterization of a soybean polygalacturonase-inhibiting protein. Planta 195:80–87
Federici L, Caprari C, Mattei B, Savino C, Di Matteo A, De Lorenzo G, Cervone F, Tsernoglou D (2001) Structural requirements of endopolygalacturonase for the interaction with PGIP (polygalacturonase-inhibiting protein). Proc Natl Acad Sci USA 98:13425
Ferrari S, Vairo D, Ausubel FM, Cervone F, De Lorenzo G (2003) Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15:93–106
Gallois P, Marinho P (1995) Leaf disc transformation using␣Agrobacterium tumefaciens-expression of heterologous genes in tobacco. Methods Mol Biol 49:39–48
Gomathi V, Gnanamanickam SS (2004) Polygalacturonase-inhibiting proteins in plant defence. Curr Sci 87:1211–1217
Hahlbrock K, Scheel D, Logemann E, Nurnberger T, Parniske M, Reinold S, Sacks WR, Schmelzer E (1995) Oligopeptide elicitor-mediated defence gene activation in cultured parsley cells. Proc Natl Acad Sci USA 92:4150–4157
Herlache TC, Hotchkiss AT, Burr TJ, Collmer A (1997) Characterization of the Agrobacterium vitis pehA gene and comparison of the encoded polygalacturonase with the homologous enzymes from Erwinia carotovora and Ralstonia solanacearum. Appl Environ Microbiol 63:338
Hood EA, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Idnurm A, Howlett BJ (2001) Pathogenicity genes of phytopathogenic fungi. Mol Plant Pathol 2:241–255
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a selective and versatile gene fusion marker in higher plants. EMBO J 13:3901–3907
Kars I, Krooshof GH, Wagemakers L, Joosten R, Benen JAE, Van Kan JAL (2005) Necrotizing activity of five Botrytis cinerea endopolygalacturonases produced in Pichia pastoris. Plant J 43:213–225
Kemp G, Stanton L, Bergmann CW, Clay RP, Albersheim P, Darvill A (2004) Polygalacturonase-inhibiting proteins can function as activators of polygalacturonase. Mol Plant Microbe Interact 17:888–894
King D, Bergmann C, Orlando R, Benen JAE, Kester HCM, Visser J (2002) Use of amide exchange mass spectrometry to study conformational changes within the endopolygalacturonase II-homogalacturonan- polygalacturonase inhibiting protein system. Biochemistry 41:10225–10233
Krooshof GH, Joosten R, Kester HCM, Kars I, Van Kan JAL, Benen JAE (2004) Endopolygalacturonases from Botrytis cinerea: biochemical properties and interaction with inhibiting proteins. XIII Botrytis symposium. Antalya, Turkey
McGarvey P, Kaper JM (1991) A simple and rapid method for screening transgenic plants. Biotechniques 11:428–432
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Pařenicová L, Benen JAE, Kester HCM, Visser J (1998) PgaE encodes a fourth member of the endoploygalacturonases of Aspergillus niger. Eur J Biochem 251:72–80
Powell ALT, Van Kan JAL, ten Have A, Visser J, Greve LC, Bennett AB, Labavitch JM (2000) Transgenic expression of pear PGIP in tomato limits fungal colonisation. Mol Plant Microbe Interact 9:942–950
Reymond P, Grunberger S, Paul K, Muller M, Farmer EE (1995) Oligogalacturonide defence signals in plants: large fragments interact with the plasma membrane in␣vitro. Proc Natl Acad Sci USA 92:4145–4149
Salzman RA, Tikhonova I, Bordelon BP, Hasegawa PM, Bressan RA (1998) Coordinate accumulation of antifungal proteins and hexoses constitutes a developmentally controlled defence response during fruit ripening in grape. Plant Physiol 117:465–472
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Shieh MT, Brown RL, Whitehead MP, Cary JW, Cotty PJ, Cleveland TE, Dean RA (1997) Molecular genetic evidence for the involvement of a specific polygalacturonase, P2c, in the invasion and spread of Aspergillus flavus in cotton bolls. Appl Environ Microbiol 63:3548–3552
Taylor RJ, Secor GA (1988) An improved diffusion assay for quantifying the polygalacturonase content of Erwinia culture filtrates. Phytopathology 78: 1101–1103
ten Have A, Mulder W, Visser J, Van Kan JAL (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol Plant Microbe Interact 11:1009–1016
ten Have A, Oude Brueil W, Wubben J, Visser J, van Kan JAL (2001) Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genet Biol 33:97–105
ten Have A, Tenberge KB, Benen JAE, Tudzynski P, Visser J, van Kan JAL (2002) The contribution of the cell wall degrading enzymes to pathogenesis of fungal plant pathogens. In: Kempken F (ed) The Mycota XI, agricultural applications. Springer-Verlag, Berlin, pp␣341–358
Wubben JP, Mulder W, ten Have A, van Kan JAL, Visser J (1999) Cloning and partial characterisation of endopolygalacturonase genes from Botrytis cinerea. Appl Environ Microbiol 65:1596–1602
York WS, Darvill AG, McNiel M, Stevenson TT, Albersheim P (1985) Isolation and characterization of plant cell walls and plant cell wall components. Methods Enzymol 118:3–40
Acknowledgements
This work was supported by grants from the South African Wine Industry (Winetech), the National research foundation of South Africa (NRF), the US Department of Energy (DOE) (DE-FG02-96ER20221) and the DOE-funded Center for Plant and Microbial Complex Carbohydrates (DE-FG02-93ER20097).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dirk A. Joubert and Ana R. Slaughter contributed equally to this work.
Rights and permissions
About this article
Cite this article
Joubert, D.A., Slaughter, A.R., Kemp, G. et al. The grapevine polygalacturonase-inhibiting protein (VvPGIP1) reduces Botrytis cinerea susceptibility in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Res 15, 687–702 (2006). https://doi.org/10.1007/s11248-006-9019-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-006-9019-1