Abstract
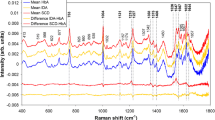
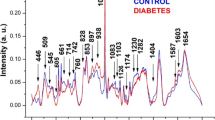
Raman spectroscopy has been proposed as a tool for diagnosis of human blood diseases aiming a quick and accurate diagnosis. Sickle cell disease arises in infancy and causes a severe anemia; thus, an early diagnosis may avoid pathological complications such as vasoocclusion, hemolytic anemia, retinopathy, cardiovascular disease, and infections. This work evaluated spectral differences between hemoglobin S (HbS) and hemoglobin A (HbA) to be used in a diagnostic model based on principal components analysis. Blood samples of patients with a previous diagnosis of sickle cell disease were hemolyzed with water, centrifuged, and the pellet was collected with a pipette. Near-infrared Raman spectra (830 nm, 200 mW) were obtained from these samples, and a model based on principal components analysis and Mahalanobis distance were used to discriminate HbA from HbS. Differences were found in the spectra of HbS and HbA, mainly in the 882 and 1,373 cm−1 (valine, HbA) and 1,547 and 1,622 cm−1 (glutamic acid, HbS). The spectral model could correctly discriminate 100 % of the samples in the correspondent groups. Raman spectroscopy was able to detect the subtle changes in the polypeptide chain (valine and glutamic acid substitution) due to the sickle cell disease and could be used to discriminate blood samples with HbS from HbA with minimum sample preparations (hemolysis with water and centrifugation).



Similar content being viewed by others
References
Center of Disease Control (CDC). http://www.cdc.gov/ncbddd/sicklecell/data.html#references Dec 2013
US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute (2009) Disease and conditions index. Sickle cell anemia: who is at risk? Bethesda, MD: US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute. http://www.nhlbi.nih.gov/health/dci/Diseases/Sca/SCA_WhoIsAtRisk.html. Dec 2013
Steinberg MH (2009) Genetic etiologies for phenotypic diversity in sickle cell anemia. Sci World J 9:46–67
Silva-Pinto AC, Angulo IL, Brunetta DM, Neves FIR, Bassi SC, De Santis GC, Covas DT (2013) Clinical and hematological effects of hydroxyurea therapy in sickle cell patients: a single-center experience in Brazil. Sao Paulo Med J 131:238–243
Salzano FM (1985) Incidence, effects and management of sickle cell disease in Brazil. Am J Pediatr Hematol Oncol 7:240–244
Gonçalves MS, Bomfim GC, Maciel E, Cerqueira I, Lyra I, Zanette A, Bomfim G et al (2003) BetaS-haplotypes in sickle cell anemia patients from Salvador, Bahia, Northeastern Brazil. Braz J Med Biol Res 36:1283–1288
Wainscoat JS, Bell JI, Thein SL, Higgs DR, Sarjeant GR, Peto TE, Weatherall DJ (1983) Multiple origin of the sickle mutations: evidence from β S-globin cluster polymorphisms. Mol Biol Med 1:191–197
Steinberg MH (2008) Sickle cell anemia, the first molecular disease: overview of molecular etiology, pathophysiology, and therapeutic approaches. Sci World J 8:1295–1324. doi:10.1100/tsw.2008.157
Pauling L, Itano H, Singer SJ, Wells IC (1949) Sickle cell anemia: a molecular disease. Science 110:543–548. doi:10.1126/science.110.2865.543
Ingram VM (1956) A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature 178:792–794
Eaton JW, Hebbel RP (1981) Pathogenesis of sickle cell disease. Pathobiol Annu 11:31–52
Hebbel RP, Eaton JW, Steinberg MH, White JG (1981) Erythrocyte/endothelial interactions and the vasocclusive severity of sickle cell disease. Prog Clin Biol Res 55:145–162
Eaton WA, Hofrichter J (1990) Sickle cell hemoglobin polymerization. Adv Protein Chem 40:63–280
Ferrone FA, Hofrichter J, Eaton WA (1985) Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol 183:611–631
Mozzarelli A, Hofrichter J, Eaton WA (1987) Delay time of HbS gelation prevents most cells from sickling in vivo. Science 237(4814):500–506
Coletta M, Hofrichter J, Ferrone FA, Eaton WA (1982) Kinetics of sickle haemoglobin polymerization in single red cells. Nature 300(5888):194–197
Trent RJ (2006) Diagnosis of the haemoglobinopathies. Clin Biochem Rev 27(1):27–38
Hoyer MD (2011) 79 Hemoglobinopathies: the how, why, and what. Division of Hematopathology Mayo Clinic Rochester, 2011 ASCP Annual Meeting, Las Vegas
Rusciano G, De Luca AC, Pesce G, Sasso A (2008) Review: Raman tweezers as a diagnostic tool of hemoglobin-related blood disorders. Sensors 8:7818–7832
Hanlon EB, Manoharan R, Koo TW, Shafer KE, Motz JT, Fitzmaurice M, Kramer JR, Itzkan I, Dasari RR, Feld MS (2000) Prospects for in vivo Raman spectroscopy. Phys Med Biol 45:R1–R59
Diem M (1993) Introduction to modern vibrational spectroscopy. Wiley, New York
Campbell ID, Durek RA (1984) Biological spectroscopy. Benjamin Cummings, New York
Berger AJ (1998) Measurement of analytes in human serum and whole blood samples by near-infrared Raman spectroscopy. PhD dissertation. Massachusetts Institute of Technology, Massachusetts
Villanueva LAE, Castro RJ, Vazquez SM, Flores GA, Ortiz LCM, Delgado AJA (2012) Raman spectroscopy of blood in-vitro. Proc. SPIE 8229, Optical diagnostics and sensing XII: toward point-of-care diagnostics and design and performance validation of phantoms used in conjunction with optical measurement of tissue IV, 82291D. doi:10.1117/12.908689
Guimarães AE, Pacheco MTT, Silveira L, Barsottini DJ, Villaverde AB, Zângaro RA (2006) Near infrared Raman spectrocopy (NIRS): a technique for doping control. Spectrosc Int J 20(4):185–194
Ryder AG, O’Connor GM, Glynn TJ (2000) Quantitative analysis of cocaine in solid mixtures using Raman spectroscopy and chemometric methods. J Raman Spectrosc 31(3):221–227
Virkler K, Lednev IK (2010) Raman spectroscopic signature of blood and its potential application to forensic body fluid identification. Anal Bioanal Chem 396:525–534
Duarte J, Pacheco MTT, Villaverde AB, Machado RZ, Zângaro RA, Silveira L (2010) Near-infrared Raman spectroscopy to detect anti-Toxoplasma gondii antibody in blood sera of domestic cats: quantitative analysis based on partial least-squares multivariate statistics. J Biomed Opt 15(4):047002
Monfared AMT, Tiwari VS, Tripathi MM, Anis H (2013) Raman spectroscopy for clinical-level detection of heparin in serum by partial least-squares analysis. J Biomed Opt 18(2):027010
Hirsch RE, Lin MJ, Vidugirus GVA, Huangi S, Friedmani JM, Nagel RL (1996) Conformational changes in oxyhemoglobin C (Glub6 3 Lys) detected by spectroscopic probing. Med J Biol Chem 271(5):372–375
Hirsch RE, Juszchq LJ, Fataliev NA, Friedman JM, Ronald L (1999) Solution-active structural alterations in ligand hemoglobins C (β6 Glu→Lys) and (β6 Glu→Val). J Biol Chem 274(20):13777–13782
Juszczak LJ, Hirsch RE, Nagel RL, Friedman JM (1998) Conformational differences in CO derivatives of HbA, HbC (Eβ6K) and HbS (Eβ6V) in the presence and absence of inositol hexaphosphate detected using ultraviolet resonance Raman spectroscopy. J Raman Spectrosc 29:963–968
Wood BR, Caspers P, Puppels GJ, Pandiancherri S, McNaughton D (2007) Resonance Raman spectroscopy of red blood cells using near-infrared laser excitation. Anal Bioanal Chem 387:1691–1703
Jolliffe IT (1995) Principal components analysis. Springer, New York, pp 1–27
The MathWorks Documentation Center. Discriminant analysis. http://www.mathworks.com/help/stats/discriminant-analysis.html#brah8i2. Feb 2013
Background: discriminant procedures. http://support.sas.com/documentation/cdl/en/statug/63347/HTML/default/viewer.htm#statug_introdisc_sect002.htm. Feb 2013
Chaiken J, Deng B, Goodisman J, Shaheen G, Bussjager RJ (2011) Analyzing near-infrared scattering from human skin to monitor change in hematocrit. J Biomed Opt 16(9):097005
Stone N, Hart Prieto MC, Crow P, Uff J, Ritchie AW (2007) The use of Raman spectroscopy to provide an estimation of the gross biochemistry associated with urological pathologies. Anal Bioanal Chem 387(5):1657–1688
Bispo JA, de Sousa Vieira EE, Silveira L, Fernandes AB (2013) Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J Biomed Opt 18(8):87004
Sigma Aldrich Catalog. http://www.sigmaaldrech/brazil.html. Jun 2013
Wajcman H, Moradkhani K (2011) Abnormal haemoglobins: detection and characterization. Indian J Med Res 134(4):538–546
Clinical Center Ltda (2008) Tabela de preços exames laboratoriais. http://www.sinescontabil.com.br/convenios/exames.htm. May 2014
NM Medical (2012) Price calculator. http://www.nmmedical.com/price-calculator-profile.htm. May 2014
Motz JT, Gandhi SJ, Scepanovic OR, Haka AS, Kramer JR, Dasari RR, Feld MS (2005) Real-time Raman system for in vivo disease diagnosis. J Biomed Opt 10(3):031113
Zhao J, Lui H, McLean DI, Zeng H (2008) Integrated real-time Raman system for clinical in vivo skin analysis. Skin Res Technol 14:484–492
Acknowledgments
L. Silveira Jr. acknowledges FAPESP (São Paulo Research Foundation, Brazil) for the partial financial support (Proc. no. 2009/018788-5 and 2012/20666-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filho, A.C.B., Silveira, L., Yanai, A.L.S. et al. Raman spectroscopy for a rapid diagnosis of sickle cell disease in human blood samples: a preliminary study. Lasers Med Sci 30, 247–253 (2015). https://doi.org/10.1007/s10103-014-1635-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1635-z