Abstract
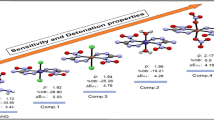
In this study, employing a new high oxygen balance energetic 3,3′-dinitro-5,5′-bis-1,2,4-triazole-1,1′-diolate anion (DNBTDO) as the bidentate ligand, NH3 and NH2NO2 as short energetic ligands, and Cu/Ni as the metal atoms, two series of novel energetic metal complexes were computationally designed. Their structures and properties were studied by density functional theory, electrostatic potential data, and molecular mechanics methods. The results showed that the designed metal complexes have high detonation performance and acceptable sensitivity: Cu/Ni(DNBTDO)(NH2NO2)2 (A3/B3) have better detonation properties and lower sensitivity than the most powerful CHNO explosive hexanitrohexaazaisowurtzitane, Cu/Ni(DNBTDO)(NH3)(NH2NO2) (A2/B2) have comparable energetic performance and sensitivity with 1,3,5,7-tetranitro-1,3,5,7-tetrazocane, Ni(DNBTDO)(NH3)2 (B1) has comparative energy level and sensitivity with 1,3,5-trinitro-1,3,5-triazinane. These five energetic metal complexes may be attractive to energetic materials researchers. Besides, both the energetic ligands and metal atoms could have a great influence on the structures, heats of formation, detonation properties, and stability of energetic metal complexes, and the effects are coupled with each other. This study may be helpful in the search for and development of new improved energetic materials.






Similar content being viewed by others
References
Bushuyev OS, Brown P, Maiti A, Gee RH, Peterson GR, Weeks BL, HopeWeeks LJ (2012) J. Am. Chem. Soc. 134:1422–1425
Wu BD, Zhang TL, Li YL, Tong WC, Zhou ZN, Zhang JG, Yang L (2013) Z. Anorg. Allg. Chem. 639:2209–2215
Ilyushin MA, Tselinskiy IV, Smirnov AV, Shugalei IV (2012) Cent Eur J Energetic Mater 9:3–16
Xu CX, Yin X, Jin X, He P, Qin J, Zhang JG, Jiao JS (2014) J. Coord. Chem. 67:2004–2015
Tang Z, Zhang JG, Liu ZH, Zhang TL, Yang L, Qiao XJ (2011) J. Mol. Struct. 1004:8–12
Wu BD, Bi YG, Li FG, Yang L, Zhou ZN, Zhang JG, Zhang TL (2014) Z. Anorg. Allg. Chem. 640:224–228
Li S, Wang Y, Qi C, Zhao XX, Zhang JC, Zhang SW, Pang SP (2013) Angew. Chem. Int. Ed. 52:14031–14035
Zhang S, Liu XY, Yang Q, Su ZY, Gao WJ, Wei Q, Xie G, Chen SP, Gao SL (2014) Chem. Eur. J. 20:7906–7910
Feng YY, Liu XY, Duan LQ, Yang Q, Wei Q, Xie GS, Chen PX, Yang W, Gao SL (2015) Dalton Trans. 44:333–339
Choi CH, Yoo HW, Goh EM, Cho SG, Jung YS (2016) J. Phys. Chem. A 120:4249–4255
Dippold AA, Klapötke TM (2013) J. Am. Chem. Soc. 135:9931–9938
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko, A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2009) Gaussian 09, Revision A. 01. Gaussian, Inc
Tao JM, Perdew JP, Starroverov VN, Scuseria GE (2003) Phys. Rev. Lett. 91:146401
Rydberg P, Olsen L (2009) J. Phys. Chem. A 113:1949–11953
Rayon VM, Valdes H, Diaz N, Suarez D (2008) J. Chem. Theory Comput. 4:243–256
Shu Y, Li H, Gao S, Xiong Y (2013) J. Mol. Model. 19:1583–1590
Sharma P, Singh HJ, Sengupta SK (2016) J. Chem. Sci. 128:1923–1932
Kamlet MJ, Jacobs S (1968) J. Chem. Phys. 48:23–35
Wang Y, Zhang JC, Su H, Li SH, Zhang SW, Pang SP (2014) J. Phys. Chem. A 118:4575–4581
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbé A (2009) Mol. Phys. 107:2095–2101
Pospíšil M, Vávra P, Koncha MC, Murray JS, Politzer P (2010) J. Mol. Model. 16:895–901
Nielson AT, Chafin AP, Christian SL, Moore DW, Nadler MP, Nissan RA, Vanderah DJ, Gilardi RD, George CF, Flippen-Anderson JL (1998) Tetrahedron 54:11793–11812
Zhang MX, Eaton PE, Gilardi R (2000) Angew. Chem. Int. Ed. 39:401–404
Mondal T, Saritha B, Ghanta S, Roy TK, Mahapatra S, Durga PM (2009) J. Mol. Struct. 897:42–47
Rice BM, Hare JJ (2002) J. Phys. Chem. A 106:1770–1783
Simpson RL, Urtiew PA, Ornellas DL, Moody GL, Scribner KJ, Hoffman DM (1997) Propellants, Explos, Pyrotech 22:249–255
Mayo SL, Olafson BD, Goddard WA (1990) J. Phys. Chem. 94:8897–8909
Wang GX, Shi CH, Gong XD, Zhu WH, Xiao HM (2009) J. Hazard. Mater. 169:813–818
Wang F, Du HC, Zhang JY, Gong XD (2011) J. Phys. Chem. A 115:11788–11795
Acknowledgements
The present work was supported by the Natural Science Foundation of Nanjing Institute of Technology (YKJ201507, CKJA201603), the National Natural Science Foundation of China (NSFC21603102), Natural Science Foundation of Jiangsu (BK20170761) and Outstanding Scientific and Technological Innovation Team in Colleges and Universities of Jiangsu Province.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wu, Q., Kou, B., Zhang, Z. et al. The search for new powerful energetic transition metal complexes based on 3,3′-dinitro-5,5′-bis-1,2,4-triazole-1,1′-diolate anion: a DFT study. J Mol Model 23, 254 (2017). https://doi.org/10.1007/s00894-017-3425-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3425-6