Abstract
In a continuing effort to further explore the use of the average local ionization energy \( \overline{\mathrm{I}}\left( \mathbf{r} \right) \) as a computational tool, we have investigated how well \( \overline{\mathrm{I}}\left( \mathbf{r} \right) \) computed on molecular surfaces serves as a predictive tool for identifying the sites of the more reactive electrons in several nonplanar defect-containing model graphene systems, each containing one or more pentagons. They include corannulene (C20H10), two inverse Stone-Thrower-Wales defect-containing structures C26H12 and C42H16, and a nanotube cap model C22H6, whose end is formed by three fused pentagons. Coronene (C24H12) has been included as a reference planar defect-free graphene model. We have optimized the structures of these systems as well as several monohydrogenated derivatives at the B3PW91/6-31G* level, and have computed their \( \overline{\mathrm{I}}\left( \mathbf{r} \right) \) on molecular surfaces corresponding to the 0.001 au, 0.003 au and 0.005 au contours of the electronic density. We find that (1) the convex sides of the interior carbons of the nonplanar models are more reactive than the concave sides, and (2) the magnitudes of the lowest \( \overline{\mathrm{I}}\left( \mathbf{r} \right) \) surface minima (the \( {{\overline{\mathrm{I}}}_{{\mathrm{S}\text{,}\min }}} \)) correlate well with the interaction energies for hydrogenation at these sites. These \( {{\overline{\mathrm{I}}}_{{\mathrm{S}\text{,}\min }}} \) values decrease in magnitude as the nonplanarity of the site increases, consistent with earlier studies. A practical benefit of the use of \( \overline{\mathrm{I}}\left( \mathbf{r} \right) \) is that a single calculation suffices to characterize the numerous sites on a large molecular system, such as graphene and defect-containing graphene models.

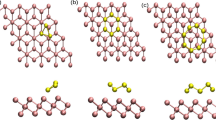
Convex 0.001 au molecular surface of hydrogenated inverse Stone-Thrower-Wales defect-containing model 4H, with the hydrogen attached to one of the central carbons fusing the two pentagons







Similar content being viewed by others
References
Sjoberg P, Murray JS, Brinck T, Politzer P (1990) Can J Chem 68:1440–1443
Koopmans TA (1934) Physica 1:104–113
Nesbet RK (1965) Adv Chem Phys 9:321–363
Politzer P, Abu-Awwad F, Murray JS (1998) Int J Quantum Chem 69:607–613
Murray JS, Seminario JM, Politzer P, Sjoberg P (1990) Int J Quantum Chem Quantum Chem Symp 24:645
Brinck T, Murray JS, Politzer P, Carter RE (1991) J Org Chem 56:2934–2936
Murray JS, Brinck T, Politzer P (1991) Int J Quantum Chem Quantum Biol Symp 40(S18):91–98
Brinck T, Murray JS, Politzer P (1991) J Org Chem 56:5012–5015
Murray JS, Brinck T, Politzer P (1992) J Mol Struct (THEOCHEM) 225:271–281
Politzer P, Murray JS, Grice ME, Brinck T, Ranganathan S (1991) J Chem Phys 95:6699–6704
Politzer P, Murray JS, Grice ME (2005) Coll Czech Chem Comm 70:550–558
Politzer P, Shields ZP-I, Bulat FA, Murray JS (2011) J Chem Theory Comput 7:377–384
Politzer P, Murray JS (2007) In: Toro-Labbé A (ed) Chemical reactivity, vol 8. Elsevier, Amsterdam, 119–137
Politzer P, Murray JS, Bulat FA (2010) J Mol Model 16:1731–1742
Brinck T, Murray JS, Politzer P (1993) Int J Quantum Chem 48:73–88
Politzer P, Murray JS, Concha MC (2002) Int J Quantum Chem 88:19–27
Politzer P, Murray JS (2012) Theor Chem Accounts 121:1114, 1–10
Murray JS, Abu-Awwad F, Politzer P (2000) J Mol Struct (THEOCHEM) 501–502:241–250
Peralta-Inga Z, Murray JS, Grice ME, Boyd S, O’Connor CJ, Politzer P (2001) J Mol Struct (THEOCHEM) 549:147–158
Bulat FA, Burgess JS, Matis BR, Baldwin JW, Macaveiu L, Murray JS, Politzer P (2012) J Phys Chem A 116:8644–8652. doi:10.1021/jp3053604
Dinadayalane TC, Murray JS, Concha MC, Politzer P, Leszczynski J (2010) J Chem Theory Comput 6:1351–1357
Saha S, Dinadayalane TC, Leszczynska D, Murray JS, Leszczynski J (2012) J Phys Chem C 116:22399–22410. doi: 10.1021/jp307090t
Barth WE, Lawton RG (1966) J Am Chem Soc 88:380–381
Hedberg L, Hedberg K, Cheng P-C, Scott LT (2000) J Phys Chem A 104:7689–7694
Slayden SW, Liebman JF (2001) Chem Rev 101:1541–1566
Scott LT, Bronstein HE, Preda DV, Ansems RBM, Bratchen MS, Hagen S (1999) Pure Appl Chem 71:209–219
Lusk MT, Carr DC (2008) Phys Rev Lett 100:175503, 1–4
Lusk MT, Carr DC (2009) Carbon 47:2226–2232
Lusk MT, Wu DT, Carr DC (2010) Phys Rev B 81:15544, 1–9
Dinadayalane TC, Leszczynski J (2010) Struct Chem 21:1156–1169
Politzer P, Lane P, Concha MC, Murray JS (2005) Microelectron Eng 81:485–493
Robinson JT, Burgess JS, Junkermeier CE, Badescu SC, Reinecke TL, Perkins FK, Salalutdniov MK, Baldwin JW, Cuthbertson JC, Shhehan PE et al (2010) Nano Lett 10:3001–3005
Teillet-Billy D, Rougeau N, Ivanovskaya VV, Sidis V (2010) Int J Quantum Chem 110:2231–2236
Hernández Rosas JJ, Ramírez Gutiérrez RE, Escobedo-Morales A, Chigo Anota E (2011) J Mol Model 17:1133–1139
Wang Y, Qian H-J, Morokuma K, Irle S (2012) J Phys Chem A 116:7154–7160
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA et al (2009) Gaussian 09, Revision A.1. Gaussian, Wallingford
Bulat FA, Toro-Labbé A, Brinck T, Murray JS, Politzer P (2010) J Mol Model 16:1679–1691
Bader RWF, Carrol MT, Cheeseman JR, Chang C (1987) J Am Chem Soc 109:7968–7979
Baskin Y, Meyer L (1955) Phys Rev 100:544
Allen FH, Kennard O, Watson DG, Brammer L, Guy Orpen A, Taylor R (1987) J Chem Soc Perk Trans II:S1-S19
Dinadayalane TC, Leszczynski J (2007) Chem Phys Lett 434:86–91
Boukhvalov DW, Katsnelson MI (2008) Nano Lett 8:4373–4379
Acknowledgments
We are thankful for the continued support and guidance that we receive from Peter Politzer, to whom this paper is dedicated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murray, J.S., Shields, Z.PI., Lane, P. et al. The average local ionization energy as a tool for identifying reactive sites on defect-containing model graphene systems. J Mol Model 19, 2825–2833 (2013). https://doi.org/10.1007/s00894-012-1693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1693-8