Abstract
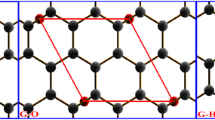
The electrical and chemical properties of graphene (C24H12), graphane (C24H24) and graphene oxide (C54H17+O+(OH)3+COOH) were studied through the density functional theory (DFT) at level of Local Density Approximation (LDA) using a model CnHm like. The optimized geometry, energy gap and chemical reactivity for the proposed carbon 2D models are reported. It was found that while the graphene and graphane structures have semiconductor behavior, the graphene oxide behaves as semi-metal. However, a transition from semi-mental to semiconductor is predicted if the carboxyl group (COOH) is removed from such structure. The chemically active sites are analyzed on the basis of the electrophilic Fukui functions for each structure.




Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Science 306:666–669
Novoselov KS, Jiang D, Schedin F, Booth TJ, Khotkevich VV, Morozov SV, Geim AK (2005) Proc Natl Acad Sci USA 102:10451–10453
Schedin F, Geim AK, Morozov SV, Hill EW, Blake P, Katsnelson MI, Novoselov KS (2007) Nat Mater 6:652–655
Serrano J, Bosak A, Arenal R, Krisch M, Watanabe K, Taniguchi T, Kanda H, Rubio A, Wirtz L (2007) Phys Rev Lett 98:095503–095504
Bolotin KI, Sikes KJ, Hone J, Strormer HL, Kim P (2008) Phys Rev Lett 101:096802–096804
Bolotin KI, Sikes KJ, Jiang Z, Klima M, Fudenberg G, Hone J, Kim P, Stormer HL (2008) Solid State Commun 146:351–355
Leenaerts O, Partoens B, Peeters FM. arXiv:0810.4056v1 [cond-mat.mtrl-sci]
Sofo JO, Chaudhari AS, Barber GD (2007) Phys Rev B 75:153401–153404
Boukhvalov DW, Katsnelson MI, Lichtenstein AI (2008) Phys Rev B 77:035427
Elias DC, Nair RR, Mohiuddin TMG, Morozov SV, Blake P, Halsall MP, Ferrari AC, Boukhvalov DW, Katsnelson MI, Geim AK, Novoselov KS (2006) Science 323:610–613
Dikin DA, Stankovich S, Zimney EJ, Piner RD, Dommett GHB, Evmenenko G, Nguyen ST, Ruoff RS (2007) Nature 448:457–460
Chigo-Anota E (2009) Sup y Vac 22:19–23
Chigo-Anota E, Salazar-Villanueva M, Hernández-Cocoletzi H. Phys Stat Solidi C in press. doi:10.1002/pssc.200983909
Chigo-Anota E, Salazar-Villanueva M, Hernández-Cocoletzi H. J Nanosci Nanotechnol in press. doi:10.1166/jnn.2011.3441
Chigo-Anota E, Salazar-Villanueva M, Hernández-Cocoletzi H (2010) Phys Stat Solidi C 7:2252–2254
Chigo-Anota E, Salazar-Villanueva M (2009) Sup y Vac 22:23–28
Delley B (1990) J Chem Phys 92:508–517
Kohn W, Becke AD, Parr RG (2006) J Phys Chem 100:12974–12980
Jones RO, Gunnarsson O (1989) Rev Mod Phys 61:689–746
Kohn W (1999) Rev Mod Phys 71:1253–1266
Perdew JP, Wang Y (1992) Phys Rev B 45:13244–13249
Delley B (1996) J Phys Chem 100:6107–6110
Delley B (2000) J Chem Phys 113:7756–7764
Foresman JB, Frisch Æ (1996) Exploring chemistry with electronic structure methods, 2nd edn. Gaussian Inc, USA, p 300
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623–11627
Frisch MJ et al. (2004) GAUSSIAN03, revision C.02. Gaussian Inc, Wallingford
Szabo A, Ostlund NS (1989) Modern quantum chemistry: introduction to advanced electronic structure theory. Mc Millan, USA, p 480
http://www.csc.fi/english/pages/g0penMol. Consulted online June 2010
Martínez JI, Cabria I, López MJ, Alonso JA (2009) J Phys Chem C 113:939–941
You YM, Ni Zh H, Yu T, Shen ZX (2008) Appl Phys Lett 93:163112–163113
Lebegue S, Klintenberg M, Eriksson O, Katsnelson MI. arXiv:0903.0310v1 [cond-mat.mtrl-sci]
Gómez-Navarro C, Thomas Weitz R, Bittner AM, Scolari M, Burghard M, Kern K (2007) Nano Lett 7:3499–3503
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H (2008) Nano Res 1:203–212
Lahaye RJWE, Jeong HK, Park CY, Lee YH (2009) Phys Rev B 79:125435–125438
Pearson RG (1963) J Am Chem Soc 85:3533–3539
Gázquez JL, Méndez F (1994) J Phys Chem 98:4591–4593
Parr RG, Yang W (1984) J Am Chem Soc 106:4049–4050
Méndez F, Gázquez JL (1994) J Am Chem Soc 116:9298–9301
López P, Méndez F (2004) Org Lett 6:1781–1783
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, USA, p 342
Lee Ch, Yang W, Parr RG (1988) J Mol Struct THEOCHEM 163:305–313
Gilardoni F, Weber J, Chermette H, Ward TR (1998) J Phys Chem A 102:3607–3613
Politzer P, Murray JS, Burat FA (2010) J Mol Model in press. doi: 10.1007/s00894-010-0709-5
Peralta-Inga Z, Murray JS, Grice ME, Boyd S, O’Connor CJ, Politzer P (2001) J Mol Struct THEOCHEM 549:147–158
Sahin H, Ataca C, Ciraci S (2009) Appl Phys Lett 95:222510–222513
Acknowledgments
This work was partially supported by Vicerrectoria de Investigación y Estudios de Posgrado-Benemérita Universidad Autónoma de Puebla (CHAE-ING10-I), Facultad de Ingeniería Química-Benemérita Universidad Autónoma de Puebla (2009-2010), Cuerpo Académico Ingeniería en Materiales (BUAP-CA-177) and Consejo Nacional de Ciencia y Tecnología, Mexico (Grant No. 0083982).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernández Rosas, J.J., Ramírez Gutiérrez, R.E., Escobedo-Morales, A. et al. First principles calculations of the electronic and chemical properties of graphene, graphane, and graphene oxide. J Mol Model 17, 1133–1139 (2011). https://doi.org/10.1007/s00894-010-0818-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-010-0818-1