Abstract
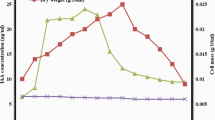
Five cell suspension lines of Catharanthus roseus resistant to 5-methyl tryptophan (5-MT; an analogue of tryptophan) were selected and characterized for growth, free tryptophan content and terpenoid indole alkaloid accumulation. These lines showed differential tolerance to analogue-induced growth inhibition by 30 to 70 mg/l 5-MT supplementation (LD50 = 7–15 mg/l). Lines P40, D40, N30, D50 and P70 recorded growth indices (i.e. percent increment over the initial inoculum weight) of 840.9, 765.0, 643.9, 585.7 and 356.5 in the absence and, 656.7, 573.9, 705.8, 489.0 and 236.0 in the presence of 5-MT after 40 days of culture, respectively. A corresponding increment in the free tryptophan level ranging from 46.7 to 160.0 μg/g dry weight in the absence and 168.0 to 468.0 μg/g dry weight in the presence was noted in the variant lines. Higher tryptophan accumulation of 368.0 and 468.0 g/g dry weight in lines N30 and P40 in 5-MT presence also resulted in higher alkaloid accumulation (0.65 to 0.90 % dry weight) in them. High-performance liquid chromatography (HPLC) analysis of the crude alkaloid extracts of the selected lines did not show the presence of any pharmaceutically important monomeric or dimeric alkaloids except catharanthine in traces in the N30 line that was also unique in terms of a chlorophyllous green phenotype. The N30 line under optimized up-scaling conditions in a 7-l stirred tank bioreactor using Murashige and Skoog medium containing 2 mg/l α-naphthalene acetic acid and 0.2 mg/l kinetin attained 18-folds biomass accumulation within 8 weeks. Interestingly, the cell biomass yield was enhanced to 30-folds if 30 mg/l 5-MT was added in the bioreactor vessel one week prior to harvest. Crude alkaloid extract of the cells grown in shake flask and this bioreactor batch also showed the formation of yellow-coloured crystals which upon 1HNMR and ESI-MS analysis indicated a phenolic identity. This crude alkaloid extract of bioreactor-harvested cells containing this compound at 50 μg/ml concentration registered 65.21, 17.75, 97.0, 100 % more total antioxidant capacity, reducing power, total phenolic content, and ferric-reducing antioxidant power, respectively, when compared with that of extracts of cells grown in shake flask cultures. The latter, however, showed 57.47 % better radical scavenging activity (DPPH) than the bioreactor-harvested cells.


Similar content being viewed by others
References
Bieleski RL, Turner NA (1966) Separation and estimation of amino acids in crude plant extracts by thin layer electrophoresis and chromatography. Ann Biochem 17:278–293
Canel C, Lopes-Cardoso MI, Whitmer S, Van der Fits L, Pasquali G, Van der Heijden R, Hoge JH, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Cho HJ, Brotherton JE, Song HS, Widhlm JM (2000) Increasing tryptophan synthesis in a forage legume Astragalus sinicus by over expressing the tobacco feedback insensitive anthranilate synthase(ASA2) gene. Plant Physiol 123:1069–1076
Chung YC, Chang CT, Chao WW, Lin CF, Chou ST (2002) Antioxidative activity and safety of the 50 % ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J Agric Food Chem 50:2454–2458
Dalby A, Tsai CY (1975) Acetic anhydride requirement in the colorimetric determination of tryptophan. Anal Biochem 63:283–285
Facchini PJ, De Luca V (2008) Opium poppy and Madagascar periwinkle: model non-model system to investigate alkaloid biosynthesis in plants. Plant J 54:763–784
Fulzele DP, Heble MR (1994) Large-scale cultivation of Catharanthus roseus cells production of ajmalicine in a 20-1-airlift bioreactor. J Biotechnol 35:1–7
Galili G, Hofgen R (2002) Metabolic engineering of amino acids and storage proteins in plants. Met Eng 4:3–11
Goddijn OJM, Pennings EJM, Van der Helm P, Schilperoort RA, Verpoorte R, Hoge JHC (1995) Overexpression of a tryptophan decarboxylase cDNA in Catharanthus roseus crown gall calluses results in increased tryptamine levels but not in increased terpenoid indole alkaloid production. Transgenic Res 4:315–323
Guirimand G, Courdavault V, Lanoue A, Mahroug S, Guihur A, Blanc N, Giglioli-Guivarch N, St-Pierre B, Burlat V (2010) Strictosidine activation in Apocynaceae: towards a “ nuclear time bomb”. BMC Plant Biol 10:182
Gupta MM, Singh DV, Tripathi AK, Pandey R, Verma RK, Singh S, Shasany AK, Khanuja SPS (2005) Simultaneous determination of vincristine, vinblastine, catharanthine and vindoline in leaves of Catharanthus roseus by high performance liquid chromatography. J Chromatogr Sci 43:450–453
Huang TK, Mc Donald KA (2009) Bioreactor engineering for recombinant protein production in plant cell suspension cultures. Biochem Eng J 45:168–184
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004) Expression of a feedback-resistant anthranilate synthase in Catharanthus roseus hairy roots provides evidence for tight regulation of terpenoid indole alkaloid levels. Biotechnol Bioeng 86:718–727
Li J, Last RL (1996) The Arabidopsis trp5 mutant has a feedback resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol 110:51–59
Luqman S, Kumar R, Kaushik S, Srivastava S, Darokar MP, Khanuja SPS (2009) Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash. Indian J Biochem Biophys 46:122–125
Meijer AH, Verpoorte R, Hoge JHC (1993) Regulation of enzymes and genes involved in terpenoid indole alkaloid biosynthesis in Catharanthus roseus. J Plant Res 3:145–164
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murata J, Roepke J, Gordon H, De Luca V (2008) The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 20:524–542
Mustafa NR, Verpoorte R (2007) Phenolic compounds in Catharanthus roseus. Phytochem Rev 6:243–258
Ndhlala AR, Moyo M, van Staden J (2010) Natural antioxidants: fascinating or mythical biomolecules? Molecules 15:6905–6930
Pietrosuik A, Furmanova M, Lata B (2007) Catharanthus roseus: micropropagation and in vitro techniques. Phytochem Rev 6:459–473
Prieto P, Pineda M, Aguilar M (1999) Spectrometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–3402
Radwanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell 7:921–934
Scott AI, Mizukami H, Lee S-L (1979) Characterization of a 5-methyltryptophan resistant strain of Catharanthus roseus cultured cells. Phytochemistry 18:795–798
Seth R, Mathur AK (2005) Selection of 5-methyltryptophan-resistant callus lines with improved metabolic flux towards terpenoid indole alkaloid synthesis in Catharanthus roseus. Curr Sci 89:544–548
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Amer J Enol Vitic 16:144–158
ten Hoopen HJG, Vangulik WM, Schlatmann JE, Moreno PRH, Vinke JL, Heijnen JJ, Verpoorte R (1994) Ajmalicine production by cell cultures of Catharanthus roseus, from shake flask to bioreactor. Plant Cell Tissue Organ Cult 38:85–91
Tozawa Y, Hasegawa H, Terakawa T, Wasaka K (2001) Characterization of rice anthranilate synthase α- subunit genes OASA1 and OASA2, tryptophan accumulation in transgenic rice expressing feedback-insensitive mutant of OASA1. Plant Physiol 126:1493–1506
van der Fits L, Memelink J (2000) ORCA3, a jasmonate responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
van der Heijden R, Jabos DJ, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:1241–1253
Verma P, Mathur AK, Shankar K (2011) Growth, alkaloid production, rol genes integration, bioreactor up-scaling and plant regeneration studies in hairy root lines of Catharanthus roseus. Plant Biosyst. doi:10.1080/11263504.2011.649797
Verma P, Mathur AK, Srivastava A, Mathur A (2012) Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloids pathway in Catharanthus roseus: a literature up-date. Protoplasma 249:255–268
Whitmer S, Van der Heijden R, Verpoorte R (2002) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of C. roseus. J Biotechnol 96:193–203
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43:27–32
Zarate R, Verpoorte R (2007) Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Photochem Rev 6:475–491
Zhao J, Verpoorte R (2007) Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev 6:435–457
Zhao J, Hu Q, Zhu WH (2001) Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microb Technol 28:673–681
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 50:5165–5170
Acknowledgments
We gratefully acknowledge the financial support received from the Council of Scientific and Industrial Research (CSIR), New Delhi to carry out this work under its Network Project (COR-002).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
1HNMR spectra, ESI+ mass spectra and UV spectra are available. (DOC 1526 kb)
Rights and permissions
About this article
Cite this article
Verma, P., Mathur, A.K., Masood, N. et al. Tryptophan over-producing cell suspensions of Catharanthus roseus (L) G. Don and their up-scaling in stirred tank bioreactor: detection of a phenolic compound with antioxidant potential. Protoplasma 250, 371–380 (2013). https://doi.org/10.1007/s00709-012-0423-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-012-0423-5