Abstract
Besides alkaloids Catharanthus roseus produces a wide spectrum of phenolic compounds, this includes C6C1 compounds such as 2,3-dihydoxybenzoic acid, as well as phenylpropanoids such as cinnamic acid derivatives, flavonoids and anthocyanins. The occurrence of these compounds in C. roseus is reviewed as well as their biosynthesis and the regulation of the pathways. Both types of compounds compete with the indole alkaloid biosynthesis for chorismate, an important intermediate in plant metabolism. The biosynthesis C6C1 compounds is induced by biotic elicitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant phenolics cover several groups of compounds such as simple phenolics, phenolic acids, flavonoids, isoflavonoids, tannins and lignins since they are defined as compounds having at least one aromatic ring substituted by at least one hydroxyl group. The hydroxyl group(s) can be free or engaged in another function as ether, ester or glycoside (Bruneton 1999). They are widely distributed in plants and particularly present in increased levels, either as soluble or cell wall-bound compounds, as a result of interaction of a plant with its environment (Matern et al. 1995).
Catharanthus roseus (L.) G.Don (Madagascar periwinkle) is a terpenoid indole alkaloids (TIAs) producing plant. In attempts to improve the production of the valuable alkaloids such as vincristine and vinblastine, several studies on C. roseus reported also the accumulation of phenolic compounds upon biotic and/or abiotic stress. The accumulation of phenolics may also affect other secondary metabolite pathways including the alkaloid pathways, as plant defense is a complex system. Elucidation of the pathways and understanding their regulation are important for metabolic engineering to improve the production of desired metabolites (Verpoorte et al. 2002). This review deals with the phytochemistry of phenolic compounds in C. roseus, their biosynthesis and its regulation.
Phytochemistry
Simple phenolics are termed as compounds having at least one hydroxyl group attached to an aromatic ring, for example catechol.
Most compounds having a C6C1 carbon skeleton, usually with a carboxyl group attached to the aromatic ring (Dewick 2002), are phenolics. C6C1 compounds in C. roseus include benzoic acid (BA) and phenolic acids derived from BA e.g. p-hydroxybenzoic acid (p-HBA), salicylic acid (SA), 2,3-dihydroxybenzoic acid (2,3-DHBA), 2,5-dihydroxybenzoic acid (2,5-DHBA), 3,4-dihydroxybenzoic acid (3,4-DHBA), 3,5-dihydroxybenzoic acid (3,5-DHBA), gallic acid (GA) and vanillic acid.
Simple phenylpropanoids are defined as secondary metabolites derived from phenylalanine, having a C6C3 carbon skeleton and most of them are phenolic acids. For example: cinnamic acid, o-coumaric acid, p-coumaric acid, caffeic acid and ferulic acid. A simple phenylpropanoid can conjugate with an intermediate from the shikimate pathway such as quinic acid to form compounds like chlorogenic acid.
Compounds having a C6C3C6 carbon skeleton such as flavonoids (including anthocyanins) and isoflavonoids, are also among the phenolic compounds in C. roseus.
The C6C1-, C6C3- and C6C3C6 compounds reported to be present in C. roseus are reviewed in Table 1.
Biosynthesis
Phenolic compounds are generally synthesized via the shikimate pathway. Another pathway, the polyketide pathway, can also provide some phenolics e.g. orcinols and quinones. Phenolic compounds derived from both pathways are quite common e.g. flavonoids, stilbenes, pyrones and xanthones (Bruneton 1999).
The shikimate pathway, a major biosynthetic route for both primary-and secondary metabolism, includes seven steps. It starts with phosphoenolpyruvate and erythrose-4-phosphate and ends with chorismate (Herrmann and Weaver 1999). Chorismate is an important branching point since it is the substrate of 5 enzymes: chorismate mutase (CM, EC 5.4.99.5), isochorismate synthase (ICS, EC 5.4.99.6), p-hydroxybenzoate synthase or chorismate pyruvate-lyase, anthranilate synthase (AS, EC 4.1.3.27) and p-aminobenzoate synthase (EC 4.1.3.38) (reviewed by Mustafa and Verpoorte 2005). These enzymes are the starting points of several pathways leading to a great diversity of secondary metabolites including phenolics. For example, CM is responsible for the formation of prephenate, the first intermediate of phenylalanine biosynthesis. In plants, phenylalanine is thought to be the general precursor of C6C1-, C6C3- and C6C3C6 compounds and their polymers such as tannins and lignins (Wink 2000). Figure 1 shows the biosynthetic pathway of some phenolics.
Biosynthesis of C6C1
In the phenylpropanoid pathway, β-oxidation of the propyl-moiety of a C6C3 results in a C6C1, the aromatic hydroxylation generally occurs more effectively at the C6C3 level than at the C6C1 level (Torsell 1997). However, it has been shown in some studies that C6C1 gallic acid and the related hydrolysable tannins are synthesized from an early intermediate of the shikimate pathway rather than from phenylalanine or tyrosine (Werner et al. 1997; Ossipov et al. 2003). Loescher and Heide (1994) showed that p-HBA is derived from the phenylalanine pathway, though it has been proposed that the presence of the chorismate pathway leading to this compound in plants is highly probable. Other C6C1 compounds such as SA and 2,3-DHBA were proven in some plants to be synthesized via the isochorismate pathway (Wildermuth et al. 2001; Budi Muljono et al. 2002; Mustafa et al. unpublished results). In microorganisms, isochorismate is a precursor of SA and 2,3-DHBA. Both are precursors of pyochelin and enterobactin, chelating agents needed by the host for survival in an environment lacking soluble iron (Fe3+) (reviewed by Verberne et al. 1999).
ICS is the enzyme responsible for conversion of chorismate into isochorismate. In C. roseus, the ICS activity was first detected in protein extracts of the cell cultures (Poulsen et al. 1991). Its activity increased after elicitation with fungal (Pythium aphanidermatum) extract, resulting in the production of 2,3-DHBA (Moreno et al. 1994a). The purification of this enzyme showed the presence of two isoforms, which require Mg2+ for enzyme activity and are not inhibited by aromatic amino acids. Isolation of its cDNA revealed the existence of only one ICS gene in this plant encoding a 64 kD protein with an N-terminal chloroplast-targeting signal. The deduced amino acid sequence shares homology with bacterial ICS and also with AS from plants (van Tegelen et al. 1999).
Some constructs containing a C. roseus cDNA clone of ICS in sense or antisense orientation were successfully transformed into C. roseus CRPM cell line (grown in Murashige & Skoog/M&S medium with growth hormones), whereas the transformation into A12A2 line (grown in M&S medium without growth hormones) failed (Talou et al. 2001). Analysis of enzyme activities of ICS, AS and CM of the ics-sense line showed an increased (about twofold) ICS activity, a relatively non-altered AS activity and inhibition of CM activity. However, the ics-antisense line revealed that there was no correlation between ics-mRNA transcription and ICS activity, since it produced a lower level of ics-mRNA but a comparable level of ICS activity compared with that of the line transformed with an empty vector after elicitation. Also, the ICS activity was similar for the non-elicited ics-sense line and the elicited empty vector line though the latter produced a much higher level of the mRNA. After elicitation, 2,3-DHBA was not detectable in the cells or medium of either CRPM wild type or empty vector line. Surprisingly, the ics-antisense line provided a higher level of 2,3-DHBA in the cells than the ics-sense line with or without elicitation, whereas much lower levels of this compound were found in the medium of both cultures. Wild type A12A2 elicited cells produced much higher level of 2,3-DHBA compared with ics-sense-and ics-antisense elicited or non-elicited cells. The presence of the growth hormones in the medium might also affect enzymatic steps downstream of ICS, which is rate limiting for either 2,3-DHBA or SA accumulation in the CRPM line (Talou et al. 2001).
A retrobiosynthetic study of 2,3-DHBA in C. roseus showed that the ICS pathway was responsible for the increased level of this compound after elicitation (Budi Muljono et al. 2002). The ICS pathway leading to 2,3-DHBA includes ICS, 2,3-dihydro-2,3-dihydroxybenzoate synthase for removing the enolpyruvyl side chain of isochorismate and 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase for the oxidation of 2,3-dihydro-DHBA to 2,3-DHBA (Young et al. 1969).
Besides 2,3-DHBA, Budi Muljono et al. (1998) reported the presence of SA in C. roseus cell cultures. SA plays different roles in plants (Raskin 1992), the most important is as signaling compound in systemic acquired resistance (SAR) (Ryals et al. 1996; Dempsey et al. 1999). Many studies dealing with SA-dependent-and/or SA-independent pathways in plant defense response have been performed in different plant species (particularly in Arabidopsis) showing the complexity of the SAR network (Shah, 2003). In microorganisms, the isochorismate pathway leading to SA involves ICS and isochorismate pyruvate-lyase (IPL). In plants, SA is thought to be derived from the phenylalanine pathway by chain shortening of a hydroxycinnamic acid derivative leading to BA. The complete pathway has not been resolved yet, though the enzyme responsible for the last step, converting BA to SA, has been characterized (Leon et al. 1995). In Arabidopsis, the enzyme ICS1 seems to be responsible for SA synthesis in SAR, it shares 57% homology with ICS from C. roseus (Wildermuth et al. 2001).
Since the ICS pathway leading to 2,3-DHBA exists in C. roseus, the existence of the ICS pathway leading to SA in the same plant is also possible. Verberne et al. (2000) proposed the presence of the ICS pathway leading to SA in plants. Both the ICS and phenylalanine pathways may occur in C. roseus and may be regulated differently for different functions as it was proposed by Wildermuth et al. (2001) with Arabidopsis. The latter group found that Arabidopsis sid2–2 mutant, unable to produce ICS1, showed increased-susceptibility for pathogens, though it still produced a small amount of SA. However, the function and regulation of two pathways can be different in each species since Chong et al. (2001) showed that the SA accumulation in elicited tobacco cells required de novo BA synthesis from trans-cinnamic acid.
Glucosylation is found to be a rapid and main catabolic route for SA in several plants, providing β-O-d-glucosylsalicylic acid and/or SA glucose ester (e.g. Lee and Raskin 1998; Dean and Mills 2004). Increased level of SA glucoside (SAG) in C. roseus A12A2-and A11 (grown in Gamborg B5 medium with 1-naphtaleneacetic acid/ NAA) cells occurred after fungal elicitation (unpublished results), whereas a lower amount of SAG was detected in the CRPM cell line. A glycoside of SA, 3-β-O-d-glucopyranosyloxy-2-hydroxybenzoic acid, was isolated from the leaves of Vinca minor L. (Nishibe et al. 1996).
In plants, 2,3-DHBA and 2,5-DHBA may also derive from SA. The roles of these compounds in plants are still not clear and it was thought that they are the products of metabolic inactivation by additional hydroxylation of the aromatic ring (El-Basyouni et al. 1964; Ibrahim and Towers 1959). Besides SA and 2,3-DHBA, the other C6C1 compounds such as BA and 2,5-DHBA were detected in a C. roseus cell suspension culture by capillary GC (Budi Muljono et al. 1998).
Shimoda et al. (2002) showed that in C. roseus cells grown in Schenk and Hildebrandt (SH) medium with 10 mM 2,4-dichlorophenoxyacetic acid (2,4-D), SA was catabolized by a hydroxylation into 2,5-DHBA (gentisic acid) followed by a glucosylation of the newly introduced phenolic hydroxyl group. The glucosyltransferase specific for gentisic acid was isolated from C. roseus cell cultures (Yamane et al. 2002). This 41 kDa protein is regioselective, transferring glucose from UDP-glucose onto the oxygen atom of the 5-hydroxyl group of this compound. It worked also for 7-hydroxyl groups of hydrocoumarins though the relative activities were low (<1.2%) compared to that for 5-hydroxyl group of gentisic acid. Optimum activity was at pH 8.0 and the enzyme was strongly inhibited by divalent cations such as Mn2+, Co2+, Zn2+ and Fe2+. Shimoda et al. (2004) isolated a novel 55 kDa hydroxylase from C. roseus cell cultures which is responsible for the hydroxylation of SA into gentisic acid. The enzyme activity was optimal at pH 7.8 and was completely inhibited by divalent cations such as Cu2+ and Hg2+.
Catharanthus roseus cell suspension culture was reported to be able to accumulate high amount of glucovanillin after 16 h incubation time with 8.2 mM of vanillin (Sommer et al. 1997). Besides, some other C6C1 compounds such as vanillyl alcohol and vanillyl alcohol-phenyl glucoside were also found as the reduction products of vanillin and glucovanillin. Observation after 12 h and 24 h feeding experiment of a C. roseus suspension culture with vanillin showed that 12 h incubation and a cells density of 10 g inoculum provided the highest amount (16% conversion) of glucovanillin (Yuana et al. 2002). The levels of vanillin and glucovanillin decreased after 24 h. The C. roseus suspension cultures were grown in M&S medium containing growth hormones (1 mg/L 2,4-D and 1 mg/l kinetin). Besides the reduction products as mentioned by Sommer et al. (1997), this group reported also the presence of other C6C1 compounds such as vanillic acid and its glucosides (glucovanillic acid). The presence of vanillic acid in C. roseus plant was reported by Proestos et al. (2005).
Biosynthesis of C6C3
Phenylalanine ammonia-lyase (PAL, EC 4.3.1.5), responsible for the conversion of phenylalanine into cinnamic acid, is the entry-point enzyme into the phenylpropanoid pathways since the reaction product is a precursor for several phenylpropanoids for example, the simple phenylpropanoids (C6C3 compounds) such as cinnamic acid, p-coumaric acid, caffeic acid, ferulic acid and sinapic acid. Besides the precursors of C6C1 compounds, simple phenylpropanoids are also precursors of other phenolics, which in many plants act as phytoalexins or phytoanticipins e.g. flavonoids, isoflavonoids, stilbenes, monolignols and lignans (Dixon 2001), or as physical barrier against pathogen infiltration e.g. the phenylpropanoid polymer: lignin (Boudet et al. 1995; Mitchell et al. 1999). Activation of PAL is considered as a marker for ongoing SAR in a plant.
By capillary gas chromatography (GC), the presence of trans-cinnamic acid was detected in an extract of a C. roseus cell suspension culture (Budi Muljono et al. 1998). A reversed phase high performance liquid chromatography (RP-HPLC) analysis of phenolic compounds in some plant extracts showed that the C. roseus extracts contained the highest amount of a C6C3 hydroxytyrosol (310 mg/100 g DW) and a C6C1 gallic acid (42 mg/100 g DW) if compared to 26 other plant extracts analyzed. Other phenolics detected from this plant extract were ferulic acid (250 mg/100 g DW) and vanillic acid (1.3 mg/100 g DW). No flavonoids were detected in this study (Proestos et al. 2005).
Cinnamate 4-hydroxylase (C4H), a cytochrome P450-dependent enzyme, is responsible for the hydroxylation at the C-4 position of cinnamic acid to form p-coumaric acid. Hotze et al. (1995) isolated the cDNA of C4H of C. roseus. The enzyme shared 75.9% identity with C4H from other plants and the transcription was induced under various stress conditions.
Using 1H-NMR spectroscopy and multivariate data analysis, Choi et al. (2004) found that increased levels of some phenolic compounds such as chlorogenic acid and polyphenols together with increased levels of some other metabolites were major discriminating factors between healthy-and phytoplasma-infected C. roseus leaves. The other metabolites present in increased levels were loganic acid, secologanin and vindoline (from TIA pathway), succinic acid, glucose and sucrose. Some proton signals were detected close to those of chlorogenic signals (shifted approximately 0.05 ppm downfield), which are assumed to be other chlorogenic acid isomers such as 4-O-caffeoylquinic acid or 5-O-caffeoylquinic acid (Choi et al. 2004). These conjugated phenylpropanoids could be the products of an enzyme catalyzing the synthesis of quinate ester from caffeoyl-CoA. Caffeoyl-CoA and p-coumaroyl-CoA in tobacco, are the best acyl group donors for shikimate and quinate (acceptors) for the reaction catalyzed by hydroxycinnamoyl-CoA:shikimate/quinate hydroxycinnamoyltransferase (Hoffmann et al. 2003). This enzyme is important for the pathway leading to 3,4-dihydroxy substituted compounds, since in Arabidopsis thaliana it has been demonstrated that C-3 hydroxylation does not occur at the free acid level as in the case of C-4 hydroxylation. In this plant for example, p-coumarate 3-hydroxylase, a cytochrome-P450 enzyme, does not accept the free acid form or the p-coumaroyl-CoA ester, but only the shikimate and quinate esters of p-coumaroyl-CoA ester act as substrates providing caffeoyl-CoA and subsequently caffeic acid by a ligase (Schoch et al. 2001).
Biosynthesis of C6C3C6
A coupling of a p-hydroxycinnamoyl-CoA with three molecules of malonyl-CoA, subsequently followed by a Claisen-like reaction by a chalcone synthase, provides a chalcone. Chalcones are precursors for a wide range of flavonoid derivatives (C6C3C6 compounds). A Michael-type nucleophilic attack of the hydroxyl group on to the α, β-unsaturated ketone of a chalcone, leads to a flavanone (e.g. naringenin from naringenin-chalcone). From flavanones, several flavonoid groups are formed, e.g. flavones, flavonols, anthocyanidins and cathechins. The members of each group are distinguished due to the different hydroxylation patterns in the two aromatic rings, methylation, glucosylation and/or dimethylation. In plants, flavonoids occur mainly as water-soluble glycosides (Dewick 2002).
The biosynthetic pathway of C6C3C6 leading to anthocyanins is one of the best-studied biosynthetic pathways in plants. One of the reasons is because dealing with colored compounds for analysis of mutants is relatively easy (reviewed by Verpoorte et al. 2002). But so far, there are not many studies about isolation of genes and enzymes involved in this pathway in C. roseus.
Some anthocyanidins and anthoxanthins in C. roseus, were first isolated from the fresh-petals by Forsyth and Simmonds (1957). Using acid-hydrolysis and separation on paper chromatography (PC), two minor anthocyanidins were identified as petunidin and malvidin. After a more complicated separation procedure employing acidic extraction, partitioning, column chromatography, re-extraction, precipitation and recrystallization, the major anthocyanidin was isolated and identified as hirsutidin. Two anthoxantins present in the flowers were identified as kaemferol and quercetin.
Nishibe et al. (1996) isolated two flavonoids: mauritianin (=kaemferol 3-O-α-l-rhamnopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside) and quercetin 3-O-α-l-rhamnopyranosyl-(1→2)-α-l-rhamnopyranosyl-(1→6)-β-d-galactopyranoside together with chlorogenic acid from the leaves of C. roseus. Whilst, from the leaves of Vinca minor L. they isolated a flavonoid kaemferol 3-O-α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranoside-7-O-β-d-glucopyranoside together with 2,3-DHBA, 3-β-d-glucopyranosyloxy-2-hydroxybenzoic acid and chlorogenic acid. The two flavonoids isolated from the leaves of C. roseus, were also isolated from the stem by Brun et al. (1999). The latter group also isolated a new flavonol glycoside syringetin from this plant.
Filippini et al. (2003) developed a stable callus of C. roseus producing anthocyanins by continuous cell-aggregate selection. A stable cell suspension cultures was obtained from this homogeneous red pigmentation calli (V32R), which contained 30% of cells accumulating anthocyanins. Similar anthocyanins were identified by ESI-MS/MS both in this cell suspension culture and in flowers of field-grown plants. They were identified as 3-O-glucosides and 3-O-(6-O-p-coumaroyl)glucosides of petunidin, malvidin and hirsutidin (see also Piovan and Filippini in this issue).
Methylations provide a variety of flavonoids including anthocyanins, which play a role in flower colors (Harborne and Williams 2000). Two cDNAs of new O-methyltransferases (OMT), CrOMT2 and CrOMT4, were isolated from C. roseus cell suspension cultures (grown in the dark) and were overexpressed in E. coli. The enzyme CrOMT4 was inactive with all substrates tested, whilst CrOMT2 was identified as a flavonoid OMT. It performs two sequential methylations at the 3′-and 5′-positions of the B-ring in myricetin (flavonol) and dihydromyricetin (dihydroflavonol), which is characteristic for C. roseus flavonol glycosides and anthocyanins (Cacace et al. 2003). Schroeder et al. (2004) used a homology based RT-PCR strategy to search for cDNAs encoding OMTs. They characterized a B-ring 4′OMT, CrOMT6, though 3′,4′-dimethylated flavonoids had not been found so far in C. roseus. They also suggested that B-ring 3′-methylation is no hindrance for dioxygenases (such as flavanone 3β-hydroxylase, flavone synthase, flavonol synthase and anthocyanidin synthase) in flavonoid biosynthesis.
Regulation
Regulation of ICS, SA- and alkaloids production
In C. roseus, a fungal elicitor induced ICS activity (Poulsen et al. 1991; Moreno et al. 1994a). The ICS product is also a precursor of naphtoquinones (reviewed by Verberne et al. 1999). A hormone such as methyl jasmonate (MeJA) induces the ICS activity for stimulating anthraquinones (AQ) synthesis in Galium mollugo cell suspension cultures. ICS affinity for chorismate is lower than of other chorismate utilizing enzymes such as CM and AS preventing a large flux of substrate into the isochorismate pathway (Leduc et al. 1997). The regulation of ICS activity is also part of the regulation of AQ production in Morinda citrifolia (Stalman et al. 2003). The ICS activity is inhibited by auxins such as NAA and 2,4-D. ICS regulation can be different in different species. For example, in Morinda citrifolia the ICS activity and AQ production were reduced when the chorismate pool decreased by blocking the sixth metabolic step of the shikimate pathway (5-enolpyruvylshikimate 3-phosphate synthase, EC 2.5.1.19) by the herbicide glyphosate, whilst the opposite situation occurred in Rubia tinctorum cells (Stalman et al. 2003).
In C. roseus, different cell cultures showed different activation or inhibition pattern for enzymes upon elicitation. Seitz et al. (1989) showed that besides the induction of the alkaloid pathway, addition of a Pythium filtrate to a cell line of C. roseus cv. Little Delicata induced PAL activity and accumulation of phenolic compounds. Whilst, Moreno et al. (1994a) found that an increased activity of ICS paralleled the accumulation of 2,3-DHBA after elicitation of C. roseus A12A2 line with Pythium aphanidermatum extract. Effects of elicitation on different metabolic pathways in this C. roseus cell line were further observed (Moreno et al. 1996). AS and TDC were induced, resulting in an increased tryptamine level in the cells. CM was not induced, PAL activity was strongly inhibited but 2,3-DHBA accumulated in the culture medium, indicating that another pathway than the phenylalanine pathway is involved for the production of this phenolic in C. roseus upon elicitation. Different amounts of Pythium extract and/or different enzyme analysis methods used, might also explain the different findings. A small amount of Pythium extract (0.5–2.5 ml) induced PAL activity but more than 2.5 ml provided reversed effects as determined by HPLC-measurement of trans-cinnamic acid, the direct product of PAL (Moreno 1995).
In our experiments for selection for high-SA producing cell lines the C. roseus A12A2 line (grown in M&S medium without growth hormones) showed the highest total SA after fungal elicitation. The C. roseus A11 line, grown in Gamborg B5 medium supplemented with NAA, produced a moderate level of total SA, whereas the lowest total SA was found in the CRPM line which was grown in M&S medium containing a combination of NAA and kinetin (10:1) (data not shown). Auxins (Woeste et al. 1999) and cytokinins (Cary et al. 1995) are known to induce ethylene synthesis in plants (e.g. Arabidopsis seedlings), but SA inhibits ethylene biosynthesis (Leslie and Romani 1986). Auxin may act antagonistically with SA (Friedmann et al. 2003). Ethylene and jasmonate (JA)/methyl jasmonate (MeJA) are signaling compounds for induced systemic resistance (ISR) (van Wees et al. 2000). Thus, the presence of growth hormones in the medium might affect the CRPM cells to generate ISR rather than SA-dependent SAR. Plant generates either SA-dependent SAR or ISR depending on the plant species, the kind of elicitors (e.g. different pathogens), wounding, kind of herbivore, abiotic stress such as UV-light, drought, salinity and stress nutrients. In general, ISR works independently from SA-dependent SAR. However, a cross talk between the SA-dependent pathways and SA-independent pathways can occur in an attacked plant (van Wees et al. 2000; Pieterse et al. 2001; Kunkel and Brooks 2002). Some genetic studies with Arabidopsis reveal that the JA-dependent pathway can inhibit the SA-dependent pathway, and vice versa. Other studies show that either SA or JA can induce certain genes involved in SAR. Some ISR expressed genes require JA and ethylene, whilst the others only JA (reviewed by Glazebrook et al. 2003). Cross talk among these pathways can occur for a fine-tuning in SAR (Shah 2003). Terpenoid indole alkaloids (TIAs) production in C. roseus is induced by MeJA (van der Fits and Memelink 2000) but auxins were found to suppress the transcription of TDC and STR (some JA-responsive genes in TIA pathway). Whilst, addition of SA (0.1 mM) provided weak inducing effects on the steady state of those mRNAs 8–24 h after treatment (Pasquali et al. 1992). Large increases in the specific content of TIAs and phenolic compounds were observed in media with high sucrose levels but lacking 2,4-D and some minerals (Knobloch 1981).
In an experiment using the C. roseus A12A2 cell suspension cultures fed with loganin and tryptamine, MeJA caused a high level of accumulation of strictosidine and ajmalicine, but SA decreased the level of ajmalicine compared to the control fed sample (El Sayed and Verpoorte 2002). This might be a result of inhibition of the JA-dependent pathway by the SA-dependent pathway. However, an increase in enzyme activities or the transcription of a/some JA-responsive gene(s) in elicited plant cells may not be seen as activation of the JA-dependent pathway (ISR) only. A cross talk between JA-and SA-dependent pathways for fine-tuning SAR could happen for example in C. roseus A12A2 cell suspension cultures elicited by Pythium extract. The elicitation increased the ICS activity and the levels of SA and 2,3-DHBA (Budi Muljono et al. 2002), but induced also AS and tryptophan decarboxylase (TDC, EC 4.1.1.28) activities, and led to the accumulation of tryptamine (Moreno et al. 1996). However, strictosidine synthase (STR, EC 4.3.3.2) activity was not significantly induced and two enzymes from the TIA pathway: isopentenyl diphosphate isomerase (IPP-isomerase) and geraniol 10-hydroxylase (G10H) were inhibited. The alkaloid ajmalicine was not increased compared with the non-elicited (control) cells, showing the limitation of TIA(s) biosynthesis by blocking the activities of some other JA-responsive genes. TDC is regulated by ORCA3 (Octadecanoid-Responsive Catharanthus AP2/ERF-domain) gene, which is induced by MeJA and elicitor (van der Fits and Memelink, 2000). In C. roseus cells, TDC expression seems not inversely related to ICS expression and biosynthesis of SA upon elicitation with Pythium.
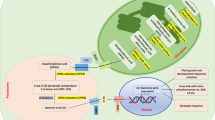
In some studies with C. roseus cell suspension cultures, auxins suppress not only TDC-but also STR expression, the level of alkaloids, the ICS activity and the level of 2,3-DHBA after Pythium elicitation as mentioned previously. Also, combination of auxin (NAA) and cytokinin (kinetin) strongly suppress the SA level in C. roseus cell suspension cultures CRPM line. Interestingly, the combination of cytokinin and ethylene strongly enhanced the expression of G10H and clearly increased the expression of the MEP pathway genes (DXS, DXR and MECS) but did no effect HMGR (belonging to the mevalonate pathway), TDC and STR expressions in C. roseus suspension cultures of C20D line. The hormones had no or little effect on the expression of these genes when they were given separately (Papon et al. 2005). The same C. roseus cell line showed a decrease in ethylene production when treated with cytokinin (Yahia et al. 1998). Combination of cytokinin-ethylene or cytokinin-auxin clearly shows different regulations for different parts of a TIA pathway. Apparently different signaling compounds can be employed and cross-talk among them can occur in the regulation of the secondary metabolite biosynthetic pathways. As discussed before, auxins also inhibited the ICS activity in Morinda citrifolia (Stalman et al. 2003) and induced by MeJA in Galium mollugo (Leduc et al. 1997) for accumulation of AQ. In C. roseus, increased levels of ICS activity paralleled the accumulation of 2,3-DHBA and SA upon a fungal elicitation. The presence of the ICS pathway leading to SA and whether ICS is a JA-responsive gene requires further study. Figure 2 summarizes the effects reported for various plant hormones and signal compounds in C. roseus cell cultures.
Summary of effects reported for various plant hormones and signal compounds in Catharanthus roseus cell cultures. A continued-line means one-step reaction. A small-dashed line means multi-step reactions. A big-dashed line with + or − indicates activation or inhibition of gene(s) expression, enzyme activity or end product level. A big-dashed line with both + and-means a concentration-dependent activation or inhibition. A strong activation or-inhibition is indicated by ++ or −−
In C. roseus seedlings, El Sayed and Verpoorte (2004) showed that MeJA was a general inducer for all alkaloids, but SA application increased also the production of serpentine and tabersonine, moreover it provided the highest level of vindoline compared to other hormone treatments. Auxins cause different effects in seedlings and suspension cell cultures, as a transient increase of TDC activity was found only in C. roseus seedlings (Aerts et al. 1992).
Sudheer and Rao (1998) reported that C6C1 compounds such as gentisic acid and 3,4-dihydroxybenzaldehyde enhanced the growth and total alkaloid content, but p-HBA provided opposite effects in C. roseus plants.
Since SA is important for signaling in SAR, cross talk between the shikimate-and phenylalanine pathway is possible. PAL up-regulation may not affect the isochorismate pathway, since ICS is not inhibited by aromatic amino acids (van Tegelen et al. 1999). The shikimate pathway exists in plastids (Herrmann and Weaver 1999) and the phenylalanine SA pathway is thought to be present in the cytosol. Metabolite transport is clearly an important factor in regulation of SA synthesis. For example, SA can be synthesized in the plastids via the ICS pathway and subsequently exported to the cytosol, or synthesized from phenylalanine in the cytosol. The presence of small amounts of SA in tobacco plants overexpressing the genes encoding the bacterial pathway for SA without plastidial signal sequence can also indicate the presence of a cytosolic pathway, which requires transport of chorismate/isochorismate out of the plastids (Verberne et al. 2000).
Regulation of PAL, phenylpropanoids-and alkaloids production
Moreno et al. (1994b) showed that UV treatment of a C. roseus cell suspension culture (A12A2 line) stopped the cell growth and increased PAL activity. Addition of 2,3-DHBA into the cell cultures induced AS, STR and slightly TDC, whilst combined treatment with UV and 2,3-DHBA, strongly induced PAL-, AS-, STR-, TDC-activity, tryptamine accumulation and inhibited growth and G10H activity. As mentioned previously, elicitation with Pythium extract on this cell line strongly inhibited PAL activity (Moreno et al. 1996), showing the different gene regulation caused by different biotic/abiotic stresses.
PAL activity increased from 4 μkat/kg to 34 μkat/kg protein when a C. roseus cell culture was exposed to 1 mM 2,2′-azobis(2-amidinopropane)-dihydrochloride (=AAPH, a free radical-generating substance) (Ohlsson et al. 1995). The cells were grown in light on a half strength Gamborg B5 medium containing 2 mg/l NAA, 0.05 mg/l kinetin and 3% sucrose. Two days after an application of 5 mM AAPH, an increase of the content of phenolic substances in the medium (from 18 mg/ml to 67 mg/ml, determined with chlorogenic acid as reference) was found. It is known, that generation of free radicals in plant cells, known as oxidative burst is part of the hypersensitive reaction (HR) as an early step before the onset of SAR (Ryals et al. 1996). Thus, exposing a plant to a free radical-generating substance can lead to SAR including PAL activation.
A recent study performed by Xu and Dong (2005) demonstrated that O −2 rather than H2O2 was found to trigger PAL activation and catharanthine synthesis in C. roseus cell cultures. The cell culture was grown in a liquid M&S medium supplemented with 2 mg/l NAA, 2 mg/l IAA, 0.1 mg/l kinetin and 3% sucrose in the dark. O −2 generated by the reaction of xanthine/xanthine oxidase, without the presence of elicitor (Aspergillus niger cell wall components), was able to activate PAL and catharanthine synthesis and to reverse the inhibitory effect of diphenylene iodonium on elicitor-induced PAL activation and catharanthine synthesis. External application of H2O2 and catalase had no effect on those plant defense responses.
The study discussed above shows the activation of PAL and the production of alkaloids upon an abiotic stress in the presence of growth hormones. Another study revealed that competition for the carbon source may occur between the phenylpropanoid pathway and TIA pathway. For example, elicitation of C. roseus cell suspension culture by biotic stress (a fungal elicitor) in the presence of trans-cinnamic acid (a PAL inhibitor) increased the alkaloid production (300% higher than non-treated cells) 72-h after treatment (Godoy-Hernandez and Loyola-Vargas 1991). Scaling up a C. roseus cell suspension culture from 250 ml to a 14-l bioreactor decreased the total alkaloid production more than 80%. But combination of osmotic stress and the inhibition of PAL activity by adding 1 mM trans-cinnamic acid into the bioreactor restored the original alkaloid amounts (Godoy-Hernandez et al. 2000). Caffeic acid and ferulic acid were found to enhance the growth and total alkaloid content in C. roseus plants, whereas p-coumaric acid showed an opposite effects (Sudheer and Rao 1998).
Regulation of C6C3C6 and alkaloid biosynthesis
Light induces the production of some anthocyanins detected as anthocyanidins (malvidin, petunidin) in a callus culture of C. roseus 21 days after inoculation (Carew and Krueger 1976). The callus culture originated from a C. roseus callus grown in the dark and which was transferred in a Gamborg agar medium (PRL 1), subcultured and then placed under 2150 lux continuous cool ray fluorescent light. Increasing light intensity and by adding a precursor like either phenylalanine or trans-cinnamic acid (100 mg/l) into the medium, increased the accumulation of the pigments. Removal of the light source inhibited pigment accumulation and increasing the sucrose concentration (2%) also decreased the accumulation.
Knobloch et al. (1982) found the same anthocyanidins in medium-induced cell suspension cultures of C. roseus. This group studied the influence of environmental factors such as medium composition and light on the accumulation of ajmalicine, serpentine, phenolics, and anthocyanins as well as on the growth rate of the cells. Transferring a 2-week-old cell suspension culture (grown in M&S medium with 2μM 2,4-D in the dark) into a 10-fold volume of an 8% aqueous sucrose solution in the dark, caused accumulation of ajmalicine, but no anthocyanins were detected after 2 weeks incubation. Continuous illumination of this medium-induced suspension cells leads to a lower level of ajmalicine but a considerable amount of the oxidation product of ajmalicine (serpentine), an increased level of phenolics and the accumulation of anthocyanins. Interestingly, only about 5% of the cells in a culture showed a high content of anthocyanins (red color). Hall and Yeoman (1986) reported that anthocyanin production in C. roseus cell cultures is determined by the percentage of producing cells. The accumulation levels in all the producing cells are very similar, pointing to a feedback inhibition mechanism controlling the anthocyanin concentration. The percentage of producing cells never exceeded 20%. A similar situation was found by microscopic analysis for the serpentine-producing cells. The optimal effect of light to stimulate the formation of anthocyanins and serpentine required low concentrations of 2,4-D, phosphate and mineral nitrogen (Knobloch et al. 1982). Quercetin was found to inhibit the growth and total alkaloid content in C. roseus plants (Sudheer and Rao 1998).
Conclusion
Either biotic or abiotic stress or a combination of both increases the production of phenolic compounds in C. roseus. Different kinds of stress may affect different parts of the SAR pathways and may determine whether SA, JA, ethylene or more than one signaling compound is employed in a plant species such as in C. roseus. A cross talk between the SA-dependent-and the SA-independent pathways may result in induction of different pathways for the production of phenolic compounds and/or other secondary metabolites. For example, biosynthesis of SA can employ either the ICS pathway or the phenylalanine pathway, which may depend on many factors including the kind of stress. This may result in e.g. activation of a part of the TIA pathway and inhibition of other parts. The results of the SAR studies in other plant species can give important information for a comparison, but one should be careful not to generalize those, because many factors determine the activation or inhibition of a pathway even within a species. The defense responses can be different for different cultivars or for intact plants, seedlings, plant cell cultures, or even cell types.
Unraveling the biosynthetic pathway of phenolic compounds like SA upon stress in C. roseus will be useful to develop strategies for increasing alkaloid production by engineering metabolic pathways in this plant. If the isochorismate pathway is responsible for the synthesis of SA necessary for SAR in the cells (as in the case of 2,3-DHBA), it is interesting to know why the induction of the ICS activity parallels the induction of TDC, which is a product of a JA-responsive gene. Elicitation with Pythium may activate both JA-and SA-regulated genes or possibly ICS is also a JA-responsive gene, as in Rubia tinctoria cells ICS is induced by MeJA in connection with the accumulation of AQ. Combinations of growth hormones such as cytokinin-ethylene activates some genes from the terpenoid pathway and the MEP pathway resulting in increased levels of ajmalicine, but had no effect on TDC and STR expressions. These results are in accordance with the finding that the terpenoid pathway is a limiting factor for alkaloid biosynthesis. Upon fungal elicitation, the activities of TDC and STR increased in parallel with the biosynthesis of SA. The SA pathway after elicitation is strongly suppressed by a combination of cytokinin-auxin.
From the various studies it is clear that the different secondary metabolites pathways are part of a complex network that is regulated by a combination of factors, including some signal compounds. For example, activation of PAL and alkaloid biosynthesis needs further investigation as competition for the carbon source between phenylpropanoid pathway and TIA pathway may occur. A better insight in the regulation of the various secondary metabolite pathways in C. roseus will thus be important. The combination of genomic, transcriptomic, proteomic and metabolomic approaches will be an important tool for unraveling the SAR controlled-pathways including the biosynthetic pathways of the desired valuable secondary metabolites.
Abbreviations
- AQ:
-
anthraquinones
- AS:
-
anthranilate synthase
- BA:
-
benzoic acid
- C4H:
-
cinnamate 4-hydroxylase
- CM:
-
chorismate mutase
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- 2,3-DHBA:
-
2,3-dihydroxybenzoic acid
- 2,3-DHBAG:
-
2,3-dihydroxybenzoic acid glucoside
- DMAPP:
-
dimethylallyl diphosphate
- DW:
-
dry weight
- DXR:
-
1-deoxy-d-xylulose 5-phosphate reductoisomerase
- DXS:
-
1-deoxy-d-xylulose 5-phosphate synthase
- ESI:
-
electro sprayed ionization
- FAB:
-
fast atomic bombardment
- GA:
-
gallic acid
- GC:
-
gas chromatography
- G10H:
-
geraniol 10-hydroxylase
- HBA:
-
hydroxybenzoic acid
- HMGR:
-
3-hydroxy-3-methylglutaryl-CoA reductase
- RP-HPLC:
-
reversed phase high performance liquid chromatography
- ICS:
-
isochorismate synthase
- IPP:
-
isopentenyl diphosphate
- ISR:
-
induced systemic resistance
- JA:
-
jasmonate
- MECS-2C :
-
methyl-d-erythritol 2,4-cyclodiphosphate synthase
- MeJA:
-
methyl jasmonate
- MEP:
-
methylerythritol phosphate
- MS:
-
mass spectrometry
- M&S:
-
Murashige & Skoog
- NMR:
-
nuclear magnetic resonance
- NAA:
-
1-naphtaleneacetic acid
- OMT:
-
O-methyltransferase
- PAL:
-
phenylalanine ammonia-lyase
- PC:
-
paper chromatography
- RT-PCR:
-
reversed transcription-polymerase chain reaction
- SA:
-
salicylic acid
- SAG:
-
salicylic acid glucoside
- SAR:
-
systemic acquired resistance
- SH:
-
Schenk and Hildebrandt
- STR:
-
strictosidine synthase
- TDC:
-
tryptophan decarboxylase
- TIA:
-
terpenoid indole alkaloid
- TLC:
-
thin layer chromatography
- UV:
-
ultra violet
References
Aerts RJ, Alarco AM, De Luca V (1992) Auxins induce tryptophan decarboxylase activity in radicles of Catharanthus roseus seedlings. Plant Physiol 100:1014–1019
Boudet AM, Lapierre C, Grima-Pettenati J (1995) Biochemistry and molecular biology of lignification. New Phytol 129:203–236
Bruneton J (1999) Pharmacognosy: phytochemistry medicinal plants, 2 edn. Intercept Ltd., Hampshire, UK, pp 227–231
Brun G, Dijoux MG, David B, Mariotte AM (1999) A new flavonol glycoside from Catharanthus roseus. Phytochemistry 50:167–169
Budi Muljono RA (2001) The isochorismate pathway as a route to 2,3-dihydroxybenzoic acid in Catharanthus roseus cell cultures. Leiden University, The Netherlands, pp 73–76
Budi Muljono RA, Looman AMG, Verpoorte R, Scheffer JJC (1998) Assay of salicylic acid and related compounds in plant cell cultures by capillary GC. Phytochem Anal 9:35–38
Budi Muljono RA, Scheffer JJC, Verpoorte R (2002) Isochorismate is an intermediate in 2,3-dihydroxybenzoic acid biosynthesis in Catharanthus roseus cell cultures. Plant Physiol Biochem 40:231–234
Cacace S, Schroeder G, Wehinger E, Strack D, Schmidt J, Schroeder J (2003) A flavonol O-methyltransferase from Catharanthus roseus performing two sequential methylations. Phytochemistry 62:127–137
Carew DP, Krueger RJ (1976) Anthocyanidins of Catharanthus roseus callus cultures. Phytochemistry 15:442
Cary AJ, Liu W, Howell SH (1995) Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol 107:1075–1082
Choi YH, Tapias EC, Kim HK, Lefeber AWM, Erkelens C, Verhoeven JThJ, Brzin J, Zel J, Verpoorte R (2004) Metabolic discrimination of Catharanthus roseus leaves infected by phytoplasma using 1H-NMR spectroscopy and multivariate data analysis. Plant Physiol 135:2398–2410
Chong J, Pierrel MA, Atanassova R, Werck-Reichhart D, Fritig B, Saindrenan P (2001) Free and conjugated benzoic acid in tobacco plants and cell cultures. Induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol 125:318–328
Dean JV, Mills JD (2004) Uptake of salicylic acid 2-O-β-D-glucose into soybean tonoplast vesicles by an ATP-binding cassette transporter-type mechanism. Physiol Plant 120:603–612
Dempsey DA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18:547–575
Dewick PM (2002) Medicinal natural products: a biosynthetic approach, 2nd edn. John Wiley & Sons Ltd, England, pp 149–151
Dixon RA (2001) Natural products and disease resistance. Nature 411:843–847
El-Basyouni SZ, Chen D, Ibrahim RK, Neish AC, Towers GHN (1964) The biosynthesis of hydroxybenzoic acids in higher plants. Phytochemistry 3:485–492
El-Sayed M, Verpoorte R (2002) Effect of phytohormones on growth and alkaloid accumulation by a Catharanthus roseus cell suspension cultures fed with alkaloid precursors tryptamine and loganin. Plant Cell Tiss Org Cult 68:265–270
El-Sayed M, Verpoorte R (2004) Growth, metabolic profiling and enzymes activities of Catharanthus roseus seedlings treated with plant growth regulators. Plant Growth Regul 44:53–58
Filippini R, Caniato R, Piovan A, Cappelletti EM (2003) Production of anthocyanins by Catharanthus roseus. Fitoterapia 74:62–67
Forsyth WGC, Simmonds NW (1957) Anthocyanidins of Lochnera rosea. Nature 180:247
Friedman H, Meir S, Halevy AH, Philosoph-Hadas S (2003) Inhibition of the gravitropic bending response of flowering shoots by salicylic acid. Plant Sci 165:905–911
Glazebrook J, Chen W, Estes B, Chang HS, Nawrath C, Metraux JP, Zhu T, Katagiri F (2003) Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J 34:217–228
Godoy-Hernandez GC, Loyola-Vargas VM (1991) Effect of fungal homogenate, enzyme inhibitors and osmotic stress on alkaloid content of Catharanthus roseus. Plant Cell Rep 10:537–540
Godoy-Hernandez GC, Vazquez-Flota FA, Loyola-Vargas VM (2000) The exposure to trans-cinnamic acid of osmotically stressed Catharanthus roseus cells cultured in a 14-L bioreactor increases alkaloid accumulation. Biotechnol Let 22:921–925
Hall RD, Yeoman MM (1986) Factors determining anthocyanin yield in cell cultures of Catharanthus roseus (L.) G.Don. New Phytol 103:33–43
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Herrmann KM, Weaver LM (1999) The shikimate pathway. Annu Rev Plant Physiol Plant Mol Biol 50:473–503
Hoffmann L, Maury S, Martz F, Geoffroy P, Legrand M (2003) Purification, cloning and properties of an acyltransferase controlling shikimate and quinate ester intermediates in phenylpropanoid metabolism. J Biol Chem 278:95–103
Hotze M, Schroeder G, Schroeder J (1995) Cinnamate 4-hydroxylase from Catharanthus roseus, and a strategy for the functional expression of plant cytochrome P450 proteins as translational fusions with P450 reductase in Escherichia coli. FEBS Lett 374:345–350
Ibrahim RK, Towers GHN (1959) Conversion of salicylic acid to gentisic acid and o-pyrocathechuic acid, all labeled with Carbon-14, in plants. Nature 184:1803
Knobloch KH, Bast G, Berlin J (1982) Medium-and light-induced formation of serpentine and anthocyanins in cell suspension cultures of Catharanthus roseus. Phytochemistry 21:591–594
Knobloch KH, Berlin J (1981) Effects of media constituents on the formation of secondary products in cell suspension cultures of Catharanthus roseus. In: Moo-Young M, Robinson CW, Vezina C (eds) Adv. Biotechnol. [Proceedings of International Ferment. Symp.], 6th, Pergamon, Toronto
Kunkel BN, Brooks DM (2002) Cross talk between signaling pathways in pathogen defense. Curr Opin Plant Biol 5:325–331
Leduc C, Birgel I, Muller R, Leistner E (1997) Isochorismate hydroxymutase from cell suspension culture of Galium mollugo. Planta 202:206–210
Lee HI, Raskin I (1998) Glucosylation of salicylic acid in Nicotiana tabacum cv. Xanthi-nc. Phytopathology 88:692–697
Leon J, Shulaev V, Yalpani N, Lawton MA, Raskin I (1995) Benzoic acid 2-hydroxylase, a soluble oxygenase from tobacco, catalyzes salicylic acid biosynthesis. Proc Natl Acad Sci USA 92:10413–10417
Leslie CA, Romani RJ (1986) Salicylic acid: a new inhibitor of ethylene biosynthesis. Plant Cell Rep 5:144–146
Loescher R, Heide L (1994) Biosynthesis of p-hydroxybenzoate from p-coumarate and p-coumaroyl-coenzyme A in cell-free extracts of Lithospermum erythrorhizon cell cultures. Plant Physiol 106:271–279
Matern U, Grimmig B, Kneusel RE (1995) Plant cell wall reinforcement in the disease-resistance response: molecular composition and regulation. Can J Bot 73:S511–S517
Mitchell HJ, Hall SA, Stratford R, Hall JL, Barber MS (1999) Differential induction of cinnamyl alcohol dehydrogenase during defensive lignification in wheat (Triticum aestivum L.): characterization of the major inducible form. Planta 208:31–37
Moreno PRH (1995) Influence of stress factors on the secondary metabolism in suspension cultured Catharanthus roseus cells. Leiden University, The Netherlands, pp 64–65
Moreno PRH, Poulsen C, van der Heijden R, Verpoorte R (1996) Effects of elicitation on different metabolic pathways in Catharanthus roseus (L.) G. Don cell suspension cultures. Enzyme Microb Technol 18:99–107
Moreno PRH, van der Heijden R, Verpoorte R (1994a) Elicitor-mediated induction of isochorismate synthase and accumulation of 2,3-dihydroxybenzoic acid in Catharanthus roseus cell suspension and shoot cultures. Plant Cell Rep 14:188–191
Moreno PRH, van der Heijden R, Verpoorte R (1994b) Induction of the secondary metabolism in Catharanthus roseus cell suspension cultures in response to UV irradiation and the addition of a benzoic acid derivative. Heterocycles 39:457–465
Mustafa NR, Verpoorte R (2005) Chorismate derived C6C1 compounds in plants. Planta 222:1–5
Nishibe S, Takenaka T, Fujikawa T, Yasukawa K, Takido M, Morimitsu Y, Hirota A, Kawamura T, Noro Y (1996) Bioactive phenolic compounds from Catharanthus roseus and Vinca minor. Natural Medicines (Tokyo) 50:378–383
Ohlsson AB, Berglund T, Komlos P, Rydstrom J (1995) Plant defense metabolism is increased by the free radical-generating compound AAPH. Free Rad Biol Med 19:319–327
Ossipov V, Salminen JP, Ossipova S, Haukioja E, Pihlaja K (2003) Gallic acid and hydrolysable tannins are formed in birch leaves from an intermediate compound of the shikimate pathway. Biochem System Ecol 31:3–16
Papon N, Bremer J, Vansiri A, Andreu F, Rideau M, Creche J (2005) Cytokinin and ethylene control indole alkaloid production at the level of the MEP/Terpenoid pathway in Catharanthus roseus suspension cells. Planta Med 71:572–574
Pasquali G, Goddijn OJM, De Waal A, Verpoorte R, Schilperoort RA, Hoge JHC, Memelink J (1992) Coordinated regulation of two indole alkaloid biosynthetic genes from Catharanthus roseus by auxin and elicitors. Plant Mol Biol 18:1121–1131
Pieterse CMJ, Ton J, Van Loon LC (2001) Cross-talk between plant defense signaling pathways: boost or burden? Ag Biotech Net 3:1–8
Poulsen C, van der Heijden R, Verpoorte R (1991) Assay of isochorismate synthase from plant cell cultures by high performance liquid chromatography. Phytochemistry 30:2873–2878
Proestos C, Chorianopoulos N, Nychas G-JE, Komaitis M (2005) RP-HPLC analysis of phenolic compounds of plant extracts. Investigation of their antioxydant capacity and antimicrobial activity. J Agricult Food Chem 53:1190–1195
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD (1996) Systemic acquired resistance. Plant Cell 8:1809–1819
Schoch G, Goepfert S, Morant M, Hehn A, Meyer D, Ullmann P, Werck-Reichhart D (2001) CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J Biol Chem 276:36566–36574
Schroeder G, Wehinger E, Lukacin R, Wellmann F, Seefelder W, Schwab W, Schroeder J (2004) Flavonoid methylation: a novel 4′-O-methyltransferase from Catharanthus roseus, and evidence that partially methylated flavanones are substrates of four different flavonoid dioxygenases. Phytochemistry 65:1085–1094
Seitz HU, Eilert U, De Luca V, Kurz WGW (1989) Elicitor-mediated induction of phenylalanine ammonia-lyase and tryptophan decarboxylase: accumulation of phenols and indole alkaloids in cell suspension cultures of Catharanthus roseus. Plant Cell Tiss Org Cult 18:71–78
Shah J (2003) The salicylic acid loop in plant defense. Curr Opin Plant Biol 6:365–371
Shimoda K, Kubota N, Sano T, Hirakawa H, Hirata T (2004) A novel hydroxylase from Catharanthus roseus participating in the hydroxylation of 2-hydroxybenzoic acid. J Biosci Bioeng 98:67–70
Shimoda K, Yamane S-y, Hirakawa H, Ohta S, Hirata T (2002) Biotransformation of phenolic compounds by the cultured cells of Catharanthus roseus. J Mol Catal B Enzym 16:275–281
Sommer J, Schroeder C, Stoeckigt J (1997) In vivo formation of vanillin glucoside. Plant Cell Tiss Org Cult 50:119–123
Stalman M, Koskamp AM, Luderer R, Vernooy JHJ, Wind JC, Wullems GJ, Croes AF (2003) Regulation of anthraquinone biosynthesis in cell cultures of Morinda citrifolia. J Plant Physiol 160:607–614
Sudheer BK, Seeta Ram Rao S (1998) Effect of phenolic compounds on growth and total alkaloid content of Catharanthus roseus (L.) G.Don. Indian J Plant Physiol 3:300–302
Talou JR, Verberne MC, Budi Muljono RA, van Tegelen LJP, Bernal BG, Lnthorst HJM, Wullems GJ, Bol JF, Verpoorte R (2001) Isochorismate synthase transgenic expression in Catharanthus roseus cell suspensions. Plant Physiol Biochem 39:595–602
Torsell KBG (1997) Natural product chemistry, a mechanistic, biosynthetic and ecological approach, 2nd edn. Apotekarsocieteten-Swedish Pharmaceutical Society, Swedish Pharmaceutical Press, Stockholm, pp 117–173
Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Van Tegelen LJP, Moreno PRH, Croes AF, Verpoorte R, Wullems GJ (1999) Purification and cDNA cloning of isochorismate synthase from elicited cell cultures of Catharanthus roseus. Plant Physiol 119:705–712
Van Wees SCM, de Swart EAM, van Pelt JA, van Loon LC, Pieterse CMJ (2000) Enhancement of induced disease resistance by simultaneous activation of salicylate-and jasmonate-dependent defense pathways in Arabidopsis thaliana. PNAS 97:8711–8716
Verberne MC, Budi Muljono RA, Verpoorte R (1999) Salicylic acid biosynthesis. In: Libbenga K, Hall M, Hooykaas PJJ (eds) Biochemistry and molecular biology of plant hormones, vol 33. Elsevier, London, pp 295–312
Verberne MC, Verpoorte R, Bol JF, Mercado-Blanco J, Linthorst HJM (2000) Overproduction of salicylic acid in plants by bacterial transgenes enhances pathogen resistance. Nat Biotechnol 18:779–783
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Werner I, Bacher A, Eisenreich W (1997) Retrobiosynthetic NMR studies with 13C-labeled glucose. J Biol Chem 272:25474–25482
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414:562–565
Wink M (2000) Biochemistry of plant secondary metabolism: annual plant reviews, vol 2. Sheffield Academic Press, Sheffield UK, pp 151–221
Woeste KE, Vogel JP, Kieber JJ (1999) Factors regulating ethylene biosynthesis in etiolated Arabidopsis thaliana seedlings. Physiol Plant 105:478–484
Xu M, Dong J (2005) O −2 from elicitor-induced oxidative burst is necessary for triggering phenylalanine ammonia-lyase activation and catharanthine synthesis in Catharanthus roseus cell cultures. Enzyme Microb Technol 36:280–284
Yahia A, Kevers C, Gaspar T, Chenieux JC, Rideau M, Creche J (1998) Cytokinins and ethylene stimulate indole alkaloids accumulation in cell suspension cultures of Catharanthus roseus by two distinct mechanisms. Plant Sci 133: 9–15
Yamane S-y, Shimoda K, Watanabe K, Hirata T (2002) Purification and characterization of gentisic acid glucosyltransferase from the cultured cells of Catharanthus roseus. J Mol Catal B: Enzym 17:59–63
Young IG, Batterham T, Gibson F (1969) The isolation, identification and properties of isochorismic acid an intermediate in the biosynthesis of 2,3-dihydroxybenzoic acid. Biochim Biophys Acta 177:389–400
Yuana, Dignum MJW, Verpoorte R (2002) Glucosylation of exogenous vanillin by plant cell cultures. Plant Cell Tiss Org Cult 69:177–182
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mustafa, N.R., Verpoorte, R. Phenolic compounds in Catharanthus roseus . Phytochem Rev 6, 243–258 (2007). https://doi.org/10.1007/s11101-006-9039-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-006-9039-8