Abstract
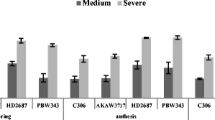
Foxtail millet (Setaria italica L.) known as a relatively drought-tolerant crop across the world is grown in arid and semi-arid regions. To the best of our knowledge, no systematic study on drought tolerance screening of foxtail millet germplasm being a drought-tolerant crop has been reported so far. To explore genetic diversity of drought-induced oxidative stress tolerance in foxtail millet, we employed lipid peroxidation measure to assess membrane integrity under stress as biochemical marker to screen 107 cultivars and classified the genotypes as highly tolerant, tolerant, sensitive, and highly sensitive. From this comprehensive screening, four cultivars showing differential response to dehydration tolerance were selected to understand the physiological and biochemical basis of tolerance mechanisms. The dehydration-tolerant cultivars (IC-403579 and Prasad) showed considerably lower levels of lipid peroxidation and electrolyte leakage as compared with dehydration-sensitive cultivars (IC-480117 and Lepakshi), indicating better cell membrane integrity in tolerant cultivars. Correspondingly, tolerant genotypes maintained higher activity of catalase (EC 1.11.1.6), ascorbate peroxidase (APX; EC 1.11.1.11), and glutathione reductase (GR; EC 1.6.4.2) across different time-course period of polyethylene glycol (PEG) treatments in comparison to sensitive ones. The above biochemical results were further validated through quantitative real-time PCR analysis of APX and GR, whose transcripts were substantially induced by PEG treatments in tolerant cultivars. These results suggest that tolerant cultivars possess wider array of antioxidant machinery with efficient ascorbate–glutathione pathway to cope with drought-induced oxidative stress.








Similar content being viewed by others
References
Abei M (1984) Catalase in vitro. Meth Enzymol 105:121–126
Alscher RG, Donahue JL, Cramer CL (1997) Reactive oxygen species and antioxidants: relationships in green cells. Physiol Plant 100:224–233
Asada K (1997) The role of ascorbate peroxidase and monodehydroascorbate reductase in H2O2 scavenging in plants. In: Scandalios JG (ed) Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor Laboratory Press, Woodbury, NY, pp 715–735
Asada K (1999) The water-water cycle in chloroplast: scavenging of active oxygens and dissipation of excess photons. Ann Rev Plant Physiol Plant Mol Biol 50:601–639
Azevedo-Neto ADD, Prisco JT, Enéas-Filho J, De Abreu CEB, Gomes-Filho E (2006) Effect 446 of salt stress on antioxidative enzymes and lipid peroxidation in leaves and 447 roots of salt-tolerant and salt-sensitive maize genotypes. Env Exp Bot 56:87–94
Barrs HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aus J Biol Sci 15:413–428
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58
Bhushan D, Pandey A, Choudhary MK, Datta A, Chakraborty S, Chakraborty N (2007) Comparative proteomics analysis of differentially expressed proteins in chickpea extracellular matrix during dehydration stress. Mol Cell Proteom 6:1868–1884
Blum A (1996) In: Edmeades GO, Banziger M, Mickelson HR, Pena-Valdivia CB (eds) Developing drought and low N-tolerant maize in proceedings of a symposium at the international maize and wheat improvement center. CIMMYT, El-Batan, pp 131–135, El-Batan, March 25–29, 1996
Bor M, Ozdemir F, Turkan I (2003) The effect of salt stress on lipid Peroxidation and antioxidants in leaves of sugar beet Beta vulgaris L. and wild beet Beta maritime L. Plant Sci 164:7–84
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 72:248–254
Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2:48–54
Chandra A, Dubey A (2010) Effect of ploidy levels on the activities of ∆-pyrroline-5-carboxylate synthetase, superoxide dismutase and peroxidase in Cenchrus species grown under water stress. Plant Physiol Biochem 48:27–34
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Demiral T, Turkan I (2005) Comparative lipid peroxidation, antioxidant systems and proline content in roots of two rice cultivars differing in salt tolerance. Env Exp Bot 53:247–257
Dionisio-Sese ML, Tobita S (1998) Antioxidative responses of rice seedlings to salinity stress. Plant Sci 135:1–9
El Hafid R, Smith DH, Karrou M, Samir K (1998) Physiological attributes associated with early-season drought resistance in spring durum wheat cultivars. Can J Plant Sci 78:227–237
Fazeli F, Ghorbanli M, Niknam V (2007) Effect of drought on biomass, protein content, lipid peroxidation and antioxidant enzymes in two sesame cultivars. Biol Plant 51:98–107
Foyer CH, Harbinson J (1994) Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux PM (eds) Causes of photo-oxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton, pp 1–4
Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione associated mechanisms of acclamatory stress tolerance and signaling. Physiol Plant 100:241–254
Garg N, Noor Z (2009) Genotypic differences in plant growth, osmotic and antioxidative defence of Cajanus cajan (L.) Millsp. modulated by salt stress. Arch Agron Soil Sci 55:3–33
González L, González-vilar M (2001) Determination of relative water content. Handbook of plant ecophysiology techniques Reigosa. Kluwer, Dordrecht, pp 207–212
Guo Z, Ou W, Lu S, Zhong Q (2006) Differential responses of antioxidative system to chilling and drought in four rice cultivars differing in sensitivity. Plant Physiol Biochem 44:828–836
Halliwell B, Gutteridge JMC (1989) Free radicals in biology and medicine, vol 2. Oxford University Press, Oxford
Hodgson RAJ, Raison JK (1991) Lipid peroxidation and superoxide dismutase activity in relation to photoinhibition induced by chilling in moderate light. Planta 185:215–219
Jayaraman A, Puranik S, Rai NK, Vidapu S, Sahu PP, Lata C, Prasad M (2008) cDNA-AFLP analysis reveals differential gene expression in response to salt stress in foxtail millet (Setaria italica L.). Mol Biotechnol 40:241–251
Jogeshwar G, Pallela R, Jakka NM, Reddy PS, Rao JV, Sreenivasulu N, Kishor PBK (2006) Antioxidative response in different sorghum species under short-term salinity stress. Acta Physiol Plant 28:465–475
Kumutha D, Ezhilmathi K, Sairam RK, Srivastava GC, Deshmukh PS, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol Plant 53:75–84
Lata C, Sahu PP, Prasad M (2010) Comparative transcriptome analysis of differentially expressed genes in foxtail millet (Setaria italica L.) during dehydration stress. Biochem Biophys Res Commun 393:720–727
Leprince O, Harren FJM, Buitink J, Alberda M, Hoekstra FA (2000) Metabolic dysfunction and unabated respiration precede the loss of membrane integrity during dehydration of germinating radicles. Plant Physiol 122:597–608
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25:402–408
Loggini B, Scartazza A, Brugnoli E, Navari-Izzo F (1999) Antioxidative defense system, pigment composition, and photosynthetic efficiency in two wheat cultivars subjected to drought. Plant Physiol 119:1091–1099
Madhusudan KV, Giridarakumar S, Ranganayakulu GS, Reddy PC, Sudhakar C (2002) Effect of water stress on some physiological responses in two groundnut (Arachis hypogea L.) cultivars with contrasting drought tolerance. J Plant Biol 29:199–202
Masood A, Shah NA, Zeeshan M, Abraham G (2006) Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides). Env Exp Bot 58:216–222
Nagy Z, Tuba Z, Zsoldos F, Erdei L (1995) CO2 exchange and water relation responses of sorghum and maize during water stress. J Plant Physiol 145:539–544
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Orvar BL, Ellis BE (1997) Transgenic tobacco plants expressing antisense RNA for cytosolic ascorbate peroxidase show increased susceptibility to ozone injury. Plant J 11:1297–1305
Pastori G, Foyer CH, Mullineaux P (1997) Low temperature-induced changes in the distribution of H2O2 and antioxidants between the bundle sheath and mesophyll cells of maize leaves. J Exp Bot 51:107–113
Sairam RK, Kumutha D, Aezhilmathi K, Chinnusamy V, Meena RC (2009) Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol Plant 53:493–504
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Silva EN, Ferreira-Silva SL, Fontenele AV, Ribeiro RV, Viégas RA, Silveira JAG (2010) Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J Plant Physiol. doi:10.1016/j.jplph.2010.03.005
Smart RE, Bingham GE (1974) Rapid estimates of relative water content. Plant Physiol 53:258–260
Smirnoff N (1995) Antioxidant systems and plant response to the environment. In: Smirnoff N (ed) Environment and plant metabolism. Bios Scientific Publishers, Oxford, pp 217–243
Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 175:408–413
Sreenivasulu N, Ramanjulu S, Ramachandra-Kini K, Prakash HS, Shekar-Shetty H, Savithri HS, Sudhakar C (1999) Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of foxtail millet with differential salt tolerance. Plant Sci 141:1–9
Sreenivasulu N, Grimm B, Wobus U, Weschke W (2000) Differential response of antioxidant compounds to salinity stress in salt-tolerant and salt-sensitive seedlings of foxtail millet (Setaria italica). Physiol Plant 109:435–442
Varghese B, Naithani SC (2008) Oxidative metabolism-related changes in cryogenically stored neem (Azadirachta indica A. Juss) seeds. J Plant Physiol 165:755–756
Wang WB, Kim YH, Lee HS, Kim KY, Deng XP, Kwak SS (2009) Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol Biochem 47:570–577
Wise RR, Naylor AW (1987) Chilling-enhanced photooxidation. Plant Physiol 83:278–282
Zhang J, Liu T, Fu J, Zhu Y, Jia J, Zheng J, Zhao Y, Zhang WG (2007) Construction and application of EST library from Setaria italica in response to dehydration stress. Genomics 90:121–131
Acknowledgments
We are grateful to the Director, National Institute of Plant Genome Research (NIPGR) for providing facilities and to University Grants Commission (U.G.C.), Govt. of India for providing UGC-SRF to Ms Charu Lata. We gratefully acknowledge the financial support from the Department of Biotechnology, Government of India (BT/IN/FRG/04/MP/2008), and IB-BMBF, Germany (IND 07/037), for carrying out the present study under the Indo-German bilateral program. We are also thankful to National Bureau of Plant Genetic Resources, Hyderabad, India, for providing the seed material.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
The authors Charu Lata and Sarita Jha contributed equally to this study.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplementary Table ESM1
(DOC 168 kb)
Supplementary Table ESM2
(DOC 29 kb)
Supplementary Table ESM3
(DOC 30 kb)
Supplementary Figure ESM1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Lata, C., Jha, S., Dixit, V. et al. Differential antioxidative responses to dehydration-induced oxidative stress in core set of foxtail millet cultivars [Setaria italica (L.)]. Protoplasma 248, 817–828 (2011). https://doi.org/10.1007/s00709-010-0257-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0257-y