Abstract
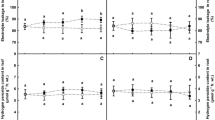
The objective of this study was to examine the role of antioxidant enzymes in waterlogging tolerance of pigeonpea (Cajanus cajan L. Halls) genotypes ICP 301 (tolerant) and Pusa 207 (susceptible). Waterlogging resulted in visible yellowing and senescence of leaves, decrease in leaf area, dry matter, relative water content and chlorophyll content in leaves, and membrane stability index in roots and leaves. The decline in all parameters was greater in Pusa 207 than ICP 301. Oxidative stress in the form of superoxide radical, hydrogen peroxide and thiobarbituric acid reactive substances (TBARS) contents initially decreased, however at 4 and 6 d of waterlogging it increased over control plants, probably due to activation of DPI-sensitive NADPH-oxidase. Antioxidant enzymes such as superoxide dismutase, ascorbate peroxidase, glutathione reductase and catalase also increased under waterlogging. The comparatively greater antioxidant enzyme activities resulting in less oxidative stress in ICP 301 could be one of the factor determining its higher tolerance to flooding as compared to Pusa 207. This study is the first to conclusively prove that waterlogging induced increase in ROS is via NADPH oxidase.
Similar content being viewed by others
Abbreviations
- APX:
-
ascorbate peroxidase
- CAT:
-
catalase
- Chl:
-
chlorophyll
- DAA:
-
days after anthesis
- DPI:
-
diphenylene iodonium chloride
- GR:
-
glutathione reductase
- MSI:
-
membrane stability index
- ROS:
-
reactive oxygen species
- RWC:
-
relative water content
- SOD:
-
superoxide dismutase
- TBARS:
-
thiobarbituric acid reactive substances
References
Aebi, H.: Catalase in vitro.-Methods Enzymol. 105: 121–126, 1984.
Agarwal, S., Sairam, R.K., Srivastava, G.C., Tyagi, A., Meena, R.C.: Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings.-Plant Sci. 169: 559–570, 2005.
Albrecht, G., Wiedenroth, E.M.: Protection against activated oxygen following re-aeration of hypoxically pre-treated wheat roots. The response of the glutathione system.-J. exp. Bot. 45: 449–455, 1994.
Arnon, D.I.: Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris.-Plant Physiol. 24: 1–15, 1949.
Beauchamp, C., Fridovich, I.: Superoxide dismutase. Improved assays and an assay applicable to acrylamide gels.-Anal. Biochem. 44: 276–287, 1971.
Biemelt, S., Keetman, U. Albrecht, G.: Re-aeration following hypoxia or anoxia leads to activation of the antioxidative defense system in roots of wheat seedlings.-Plant Physiol. 116: 651–658, 1998.
Biemelt, S., Keetman, U., Mock, H.P., Grimm, B.: Expression and activity of isoenzymes of superoxide dismutase in wheat roots in response to hypoxia and anoxia.-Plant Cell Environ. 23: 135–144, 2000.
Blokhina, O.B., Chirkova, T.V., Fagerstedt, K.V.: Anoxic stress leads to hydrogen peroxide formation in plant cells.-J. exp. Bot. 52: 1–12, 2001.
Blokhina, O.B., Fagerstedt, K.V., Chirkova, T.V.: Relationships between lipid peroxidation and anoxia tolerance in a range of species during post-anoxic reaeration.-Physiol. Plant. 105: 625–632, 1999.
Bowler, C., Montague, M.V., Inze, D.: Superoxide dismutase and stress tolerance.-Annu. Rev. Plant Physiol. Plant mol. Biol. 43: 83–116, 1992.
Chaitanya, K.S.K., Naithani, S.C.: Role of superoxide, lipid peroxidation and superoxide dismutase in membrane perturbation during loss of viability in seeds of Shorea robusta Gaertnf.-New Phytol. 126: 623–627, 1994.
Chirkova, T.V., Novitskaya, L.O., Blokhina, O.B.: Lipid peroxidation and antioxidant systems under anoxia in plants differing in their tolerance to oxygen deficiency.-Russ. J. Plant Physiol. 45: 55–62, 1998.
Collaku, A., Harrison, S.A.: Loses in wheat due to waterlogging.-Crop Sci. 42: 444–450, 2002.
Crawford, R.M.M., Braendle, R.: Oxygen deprivation stress in a changing environment.-J. exp. Bot. 47: 145–159, 1996.
Crawford, R.M.M., Walton, J.C., Wollenweber-Ratzer, B.: Similarities between post-ischaemic injury to animal tissues and post anoxic injury in plants.-Proc. roy. Soc. Edinburgh 102B: 325–332, 1994.
De Carvalho, M.C.C.G., Da Silva, D.C.G., Ruas, P.M., Medri, M.E., Ruas, E.A., Ruas, C.F.: Flooding tolerance and genetic diversity in populations of Luehea divaricata.-Biol. Plant. 52: 771–774, 2008.
Dhindsa, R.A., Plumb-Dhindsa, P., Thorpe, T.A.: Leaf senescence correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase.-J. exp. Bot. 126: 93–101, 1981.
Drew, MC.: Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia.-Annu. Rev. Plant Physiol. Plant mol. Biol. 48: 223–250, 1997.
Else, M.A., Davies, W.S., Malone, M., Jackson, M.S.: A negative hydraulic message from oxygen-deficient roots of tomato plant?-Plant Physiol. 109: 1017–1024, 1995.
Elstner, E.F.: Metabolism of activated oxygen species.-In: Davies, D.D. (ed.): The Biochemistry of Plants. Biochemistry of Metabolism. Vol. 11. Pp. 253–315. Academic Press, San Diego 1986.
Fukao, T., Bailey-Serres, J.: Plant responses to hypoxia is survival a balancing act.-Trends Plant Sci. 9: 449–456, 2004.
Gambrell, R.P., Patrick, W.H.: Chemical and microbiological properties of anaerobic soils and sediments.-In: Hook, D.D., Crawford, R.M.M. (ed.): Plant Life in Anaerobic Environments. Pp. 375–423. Ann Arbor Scientific Publications, Ann Arbor 1978.
Heath, R.L., Packer, L.: Photoperoxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation.-Arch. Biochem. Biophys. 125: 189–198, 1968.
Hiscox, J.D., Israelstam, G.F.: A method for extraction of chloroplast from leaf tissue without maceration.-Can. J. Bot. 57: 1332–1334, 1979.
Jackson, M.B., Herman, B. Goodenogh, A.: An examination of the importance of ethanol in causing injury to flooded plants.-Plant Cell Environ. 5: 163–172, 1982.
Jackson, M.B., Drew, M.C.: Effects of flooding on growth and metabolism of herbaceous plants.-In: Kozlowski, T.T. (ed.): Flooding and Plant Growth. Pp. 47–128. Academic Press, Orlando 1984.
Kalashnikov, Yu.E., Balakhnina, T.I., Zakrzhevsky, D.A.: Effect of soil hypoxia on activation of oxygen and the system of protection from oxidative destruction in roots and leaves of Hordeum vulgare.-Russ. J. Plant Physiol. 41: 583–588, 1994.
Kramer, P.J., Jackson, W.T.: Causes of injury to flooded tobacco plants.-Plant Physiol. 29: 241–245, 1954.
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4.-Nature 227: 680–685, 1970.
Min, X.J., Bartholomew, D.P.: Effects of flooding and drought on ethylene metabolism, titratable acidity and fruiting of pineapple.-Acta Hort. 666: 135–148, 2005.
Monk, L.S., Fagerstedt, K.V., Crawford, R.M.M.: Superoxide dismutase as an anaerobic polypeptide-a key factor in recovery from oxygen deprivation in Iris pseudacorus?-Plant Physiol. 85: 1016–1020, 1987.
Monk, L.S., Fagerstedt, K.V., Crawford, R.M.M.: Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress.-Physiol Plant. 76: 456–459, 1989.
Naidoo, G.: Effects of flooding on leaf water potential and stomatal resistance in Bruguiera gymporrhiza (L.) Lam.-New Phytol. 93: 369–376, 1983.
Nakano, Y., Asada, K.: Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts.-Plant Cell Physiol. 22: 867–880, 1981.
Oberson, J., Pavelic, D., Braendle, R., Rawler, A.: Nitrate increases membrane stability of potato cells under anoxia.-J. Plant Physiol. 155: 792–794, 1999.
Rao, M.V., Paliyath, G., Ormrod, D.P. Murr, D.P., Watkins, C.B.: Influence of salicylic acid on H2O2 production, oxidative stress and H2O2 metabolizing enzymes.-Plant Physiol. 115: 137–149, 1997.
Rawyler, A., Arpagaus, S., Braendle, R.: Impact of oxygen stress and energy availability on membrance stability of plant cells.-Ann. Bot. 90: 499–507, 2002.
Richard, B., Couce, I., Raymond, P., Saglio, P.H., Saint-Ges, V., Pradet, A.: Plant metabolism under hypoxia and anoxia.-Plant Physiol. Biochem. 32: 1–10, 1994.
Sairam, R.K.: Effect of moisture stress on physiological activities of two contrasting wheat genotypes.-Indian J. exp. Biol. 32: 594–593, 1994.
Sairam, R.K., Kumutha, D., Ezhilmathi, K., Deshmukh, P.S., Srivastava, G.C.: Physiology and biochemistry of waterlogging tolerance in plants.-Biol. Plant. 52: 401–412, 2008.
Sairam, R.K., Srivastava, G.C.: Water stress tolerance of wheat (Triticum aestivum L.): Variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes.-J. Agron. Crop Sci. 186: 63–70, 2001.
Sairam, R.K., Rao, K.V., Srivastava, G.C.: Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration.-Plant Sci. 163: 1037–1046, 2002.
Sairam, R.K., Srivastava, G.C., Saxena, D.C.: Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes.-Biol. Plant. 43: 245–251, 2000.
Sandalio, L.M., Palma, P.M., Del-Rio, L.A.: Localization of manganese superoxide dismutase in peroxisomes isolated from Pisum sativum L.-Plant Sci. 51: 1–8, 1987.
Singh, K., Sharma, S.P., Singh, T.K., Singh, Y.: Effect of waterlogging on growth, yield and nutrient concentration of black gram and green gram under subtropical condition of Varanasi.-Ann. agr. Res. 7: 169–177, 1986.
Smith, I.K., Vierheller, T.L., Thorne, C.A.: Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid).-Anal. Biochem. 175: 408–413, 1988.
Sorte, N.V., Deotah, R.D., Meshram, J.H., Chanekar, M.A.: Tolerance of soybean cultivars of waterlogging at various growth states.-J. Soil Crops 6: 68–72, 1996.
Tadege, M., Dupuis, I., Kuhlemeier, C.: Ethanolic fermentation: new functions for an old pathway.-Trends Plant Sci. 4: 320–325, 1999.
Ushimaru, T., Maki, Y., Sano, S., Koshiba, K., Asada, K., Tsuji, H.: Induction of enzymes involved in the ascorbate-dependent antioxidative system, namely ascorbate peroxidase, mono dehydroascorbate reductase and dehydroascorbate reductase, after exposure to air of rice (Oryza sativa) seedlings germinated under water.-Plant Cell Physiol. 38: 541–549, 1997.
Van Toai, T.T., Bolles, C.S.: Postanoxic injury in soybean (Glycine max) seedlings.-Plant Physiol. 97: 588–592, 1991.
Vartapetian, B.B., Jackson, M.B.: Plant adaptations to anaerobic stress.-Ann. Bot. 79(Suppl. A): 3–20, 1997.
Weatherley, P.E.: Studies in the water relations of cotton plants. I. The field measurement of water deficit in leaves.-New Phytol. 49: 81–97, 1950.
Yan, B., Dai, Q., Liu, X., Huang, S., Wang, Z.: Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves.-Plant Soil 179: 261–268, 1996.
Yu, Q., Rengel, Z.: Drought and salinity differentially influence activities of superoxide dismutase in narrow-leafed lupines.-Plant Sci. 142: 1–11, 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumutha, D., Ezhilmathi, K., Sairam, R.K. et al. Waterlogging induced oxidative stress and antioxidant activity in pigeonpea genotypes. Biol Plant 53, 75–84 (2009). https://doi.org/10.1007/s10535-009-0011-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-009-0011-5