Abstract
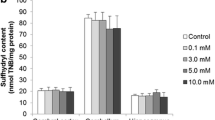
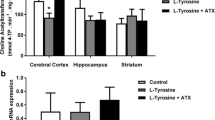
In the present study, we investigate the in vitro effect of hypoxanthine on acetylcholinesterase and butyrylcholinesterase activities in the hippocampus, striatum, cerebral cortex and serum of 15-, 30- and 60-day-old rats. Furthermore, we also evaluated the influence of antioxidants, namely α-tocopherol (trolox) and ascorbic acid, and allopurinol to investigate the possible participation of free radicals and uric acid in the effects elicited by hypoxanthine on these parameters. Acetylcholinesterase and butyrylcholinesterase activities were determined according to Ellman et al. (Biochem Pharmacol 7:88–95, 1961), with some modifications. Hypoxanthine (10.0 μM), when added to the incubation medium, enhanced acetylcholinesterase activity in the hippocampus and striatum of 15- and 30-day-old rats and reduced butyrylcholinesterase activity in the serum of 60-day-old rats. The administration of allopurinol and/or antioxidants partially prevented the alterations caused by hypoxanthine in acetylcholinesterase and butyrylcholinesterase activities in the cerebrum and serum of rats. Data indicate that hypoxanthine alters cholinesterase activities, probably through free radicals and uric acid production since the alterations were prevented by the administration of allopurinol and antioxidants. It is presumed that the cholinesterase system may be associated, at least in part, with the neuronal dysfunction observed in patients affected by Lesch–Nyhan disease. In addition, although extrapolation of findings from animal experiments to humans is difficult, it is conceivable that these vitamins and allopurinol might serve as an adjuvant therapy to avoid progression of brain damage in patients affected by this disease.







Similar content being viewed by others
References
Bajgar J (2004) Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 38:151–216
Bavaresco CS, Chiarani F, Matte C, Wajner M, Netto CA, Wyse ATS (2005) Effect of hypoxanthine on Na+, K+-ATPase activity and some parameters of oxidative stress in rat striatum. Brain Res 1041:198–204
Bavaresco CS, Chiarani F, Duringon E, Ferro MM, Cunha CD, Netto CA, Wyse ATS (2007a) Intrastriatal injection of hypoxanthine reduces striatal serotonin content and impairs spatial memory performance in rats. Metab Brain Dis 22:67–76
Bavaresco CS, Chiarani F, Wannmacher CM, Netto CA, Wyse ATS (2007b) Intrastriatal hypoxanthine reduces Na(+), K (+)-ATPase activity and induces oxidative stress in the rats. Metab Brain Dis 22:1–11
Bavaresco CS, Chiarani F, Kolling J, Ramos DB, Cognato GP, Bonan CD, Bogo MR, Sarkis JJ, Netto CA, Wyse AT (2008) Intrastriatal injection of hypoxanthine alters striatal ectonucleotidase activities: a time-dependent effect. Brain Res 1239:198–206
Benzi G, Moretti A (1998) Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease? Eur J Pharmacol 346:1–13
Bradford MM (1976) A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burton GW, Wronska U, Stone L, Foster DO, Ingold KU (1990) Biokinetics of dietary RRR-α-tocopherol in the male guinea-pig at three dietary levels of vitamin C and two levels of vitamin E. Lipids 25:199–210
Darvesh S, Hopkins D, Geula C (2003) Neurobiology of butyrylcholinesterase. Nat Rev Neurobiol 4:131–138
Delwing D, Chiarani F, Delwing D, Bavaresco CS, Wannmacher CMD, Wajner M, Wyse ATS (2003) Proline reduces acetylcholinesterase activity in cerebral cortex of rats. Metab Brain Dis 18:79–86
Dubois B, Pillon B (1995) Do cognitive changes of Parkinsons disease result from dopamine depletion? J Neural Transm 45:27–34
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fujimori S (1996) PRPP synthetase superactivity. Nihon Rinsho 54:3309–3314
Garcia-Alloza M, Gil-Bea FJ, Diez-Ariza M, Chen CP, Francis PT, Lasheras B, Ramirez MJ (2005) Cholinergic–serotonergic imbalance contributes to cognitive and behavioral symptoms in Alzheimer’s disease. Neuropsychologia 43:442–449
Geula C, Darvesh S (2004) Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer’s disease. Drugs Today 40:711–721
Geula C, Mesulam MM, Kuo CC, Tokuno H (1995) Postnatal development of cortical acetylcholinesterase-rich neurons in the rat brain: permanent and transient patterns. Exp Neurol 134:157–178
Hagan JJ, Alpert JE, Morris RG, Iversen SD (1983) The effects of central catecholamine depletions on spatial learning in rats. Behav Brain Res 9:83–104
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, London
Harkness RA, McCreanor GM, Watts RW (1988) Lesch–Nyhan syndrome and its pathogenesis: purine concentrations in plasma and in urine with metabolite profiles in CSF. J Inher Metab Dis 11:239–252
Henderson JF (1968) Possible functions of hypoxanthine–guanine phosphoribosyltransferase and their relation to the biochemical pathology of the Lesch–Nyhan syndrome. Fed Proc 27:1075–1077
Henderson VW, Watt L, Buckwalter JG (1996) Cognitive skills associated with estrogen replacement in women with Alzheimer’s disease. Psychoneuroendocrinology 21:421–430
Hong YS, Lee MJ, Kim KH, Lee SH, Lee YH, Kim BG, Jeong B, Yoon HR, Nishio H, Kim JY (2004) The C677 mutation in methylene tetrahydrofolate reductase gene: correlation with uric acid and cardiovascular risk factors in elderly Korean men. J Korean Med Sci 19:209–213
Jarasch ED, Grund C, Bruder G, Heid HW, Keenan TW, Franke WW (1981) Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell 25:67–82
Jinnah HA, Friedmann T (2001) Lesch Nyhan disease and it variants. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 2537–2569
Klein AS, Joh JW, Rangan U, Wang D, Bulkley GB (1996) Allopurinol: discrimination of antioxidant from enzyme inhibitory activities. Free radical. Biol Med 21:713–717
Lesch M, Nyhan WL (1964) A familial disorder of uric acid metabolism and central nervous system function. Am J Med 36:561–570
Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, Goldstein M, Shibuya M, Kelley WN, Fox IH (1981) Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch–Nyhan syndrome. N Engl J Med 305:1106–1111
Loewi O (1921) Uberhumerole ubertragbarkeit der herznervenwirkung. I Mitteilung Pflugers Arch 189:239–242
Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM (1993) Molecular and cellular biology of cholinesterases. Prog Neurobiol 41:31–91
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45:117–127
Mesulam MM, Guillozet A, Shaw P, Levey A, Duysen EG, Lockridge O (2002) Acetylcholinesterase knockouts establish central cholinergic pathways and can use butyrylcholinesterase to hydrolyse acetylcholine. Neuroscience 170:627–639
Mura A, Feldon J (2003) Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Mov Disord 18:860–871
Myhrer T (2000) Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Rev 41:268–287
Nyhan WL (2005) Inherited hyperuricemic disorders. Contrib Nephrol 147:22–34
Nyhan WL, Oliver WJ, Lesch M (1965) A familial disorder or uric acid metabolism and central nervous system function II. J Pediatr 67:439–444
O′Brien KK, Saxby BK, Ballard CG, Grace J, Harrington F, Ford GA, O′Brien JT, Swan AG, Fairbairn AF, Wesnes K, del Ser T, Edwardson JA, Morris CM, McKeith IG (2003) Regulation of attention and response to therapy in dementia by butyrylcholinesterase. Pharmacogenetics 13:231–239
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999) Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 56:303–308
Puig JG, Mateos FA (1993) The biochemical basis of HGPRT deficiency. In: Gresser U (ed) Molecular genetics, biochemistry and clinical aspects of inherited disorders of purine and pyrimidine metabolism. Springer, New York, pp 12–26
Rijksen G, Staal GEJ, Van der Vlist MJM, Beerner FA, Troost J, Gutensohn W, Van Laarhoven JPRM, De Bruyn CHMM (1981) Partial hypoxanthine-guanine phosphoribosyl transferase deficiency with full expression of the Lesch–Nyhan syndrome. Hum Genet 57:39–47
Rosenbloom RM, Henderson JF, Caldwell IC, Kelley WN, Seegmiller JE (1967) Inherited disorder of purine metabolism. JAMA 202:175–177
Saito Y, Takashima S (2000) Neurotransmitter changes in the pathophysiology of Lesch–Nyhan syndrome. Brain Dev 22:S122–S131
Silva CG, Bueno ARF, Schuck PF, Leipnitz G, Ribeiro CA, Rosa RB, Dutra Filho CS, Wyse AT, Wannmacher CM, Wajner M (2004) Inhibition of creatine kinase activity from rat cerebral cortex by D-2-hydroxyglutaric acid in vitro. Neuchem Int 44:45–52
Silver A (1974) The biology of cholinesterases. North-Holland, Amsterdam
Srivastava T, O’Neill JP, Dasouki M, Simckes AM (2002) Childhood hyperuricemia and acute renal failure resulting from a missense mutation in the HPRT gene. Am J Med Gene 108:219–222
Stefanello FM, Monteiro SC, Matté C, Scherer EBS, Netto CS, Wyse ATS (2007) Hypermethioninemia increases cerebral acetylcholinesterase activity and impairs memory in rats. Neurochem Res 32:1868–1874
Torres RJ, Puig JG (2007) Hypoxanthine-guanine phosphoribosyltransferase (HPRT) deficiency: Lesch–Nyhan syndrome. Orphanet J Rare Dis 8:48
Tsakiris S, Angelogianni P, Schulpis KH, Stavridis JC (2000) Protective effect of l-phenylalanine on rat brain acetylcholinesterase inhibition induced by free radicals. Clin Biochem 33:103–106
Wessler I, Kirkpatrick CJ (2008) Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 154:1558–1571
Wyse ATS, Zugno AI, Streck EL, Matte C, Calcagnotto T, Wannmacher CMD, Wajner M (2002) Inhibition of Na+, K+-ATPase activity in hippocampus of rats subjected to acute administration of homocysteine is prevented by vitamins E and C treatment. Neurochem Res 27:1685–1689
Wyse ATS, Stefanello FM, Chiarani F, Delwing D, Wannmacher CMD, Wajner M (2004) Arginine administration decreases cerebral cortex acetylcholinesterase and serum butyrylcholinesterase probably by oxidative stress induction. Neurochem Res 29:385–389
Yamamoto T, Moriwaki Y, Takahashi S (2005) Effect of ethanol on metabolism of purine bases (hypoxanthine, xanthine, and uric acid). Clin Chim Acta 356:35–57
Acknowledgments
This work was supported by grants from Universidade Regional de Blumenau and PIBIC/CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wamser, M.N., Leite, E.F., Ferreira, V.V. et al. Effect of hypoxanthine, antioxidants and allopurinol on cholinesterase activities in rats. J Neural Transm 120, 1359–1367 (2013). https://doi.org/10.1007/s00702-013-0989-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-013-0989-x