Abstract—
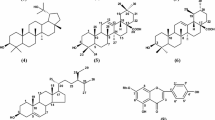
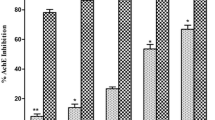
Model phenanthrene seco-alkaloids (seco-glaucine and seco-boldine) obtained in the medium of subcritical water (SBW) from plant aporphine alkaloids have been studied for the first time as antioxidants and inhibitors of acetylcholinesterase (AChE). Antioxidant activities (in vitro) of model aporphine and phenanthrene alkaloids: boldine, seco-boldine, glaucine and seco-glaucine (BD, s-BD, GL and s-GL) were studied in the reaction with a stable free radical DPPH (1,1-diphenyl-2-picrylhydrazyl). In vivo, antioxidant activity was determined in a bioluminescent test system using genetically modified E. coli strains. In the experiments in vitro (DPPH test) and in vivo (biotest), phenanthrene alkaloids s-GL and s-BD demonstrate the higher antioxidant activity than their aporphine precursors GL and BD. The anticholinesterase activity of alkaloids and their phenanthrene seco-isomers was studied (in vitro) using Ellman’s method with minor modifications. The data on the inhibitory activity of the AChE enzyme with aporphine and phenanthrene alkaloids expressed as IC50 values obtained from dose–response curves demonstrate that the inhibitory activity for seco-boldine (IC50 = 0.21 mM) and seco-glaucine (IC50 = 0.04 mM) is higher than for the initial aporphine alkaloids boldine (IC50 = 0.29 mM) and glaucine (IC50 = 0.44 mM), respectively. Thus, it has been shown that phenanthrene alkaloids obtained in SBW exhibit the higher antioxidant activity and the better inhibitory AChE activity than their aporphine precursors.






Similar content being viewed by others
REFERENCES
Shamma, M., and Moniot, J.I., Isoquinoline Alkaloids Research 1972–1977, New York: Plenum Press, 1978.
Gabbasov, T.M., Tsyrlina, E.M., Yunusov, M.S., Teslenko, V.V., Salokhin, A.V., Sabutskii, Y.E., and Gorovoi, P.G., Alkaloids from Aconitum neosachalinense, Chem. Nat. Compd., 2014, vol. 50, no. 6, pp. 1156–1157. https://doi.org/10.1007/s10600-014-1190-7
O’Brien, P., Carrasco-Pozo, C., and Speisky, H., Boldine and its antioxidant or health-promoting properties, Chem.-Biol. Interact., 2006, vol. 159, no. 1, pp. 1–17. https://doi.org/10.1016/j.cbi.2005.09.002
Vitorović-Todorović, M.D., Juranić, I.O., Mandić, L.M., and Drakulić, B.J., 4-aryl-4-oxo-N-phenyl-2-aminylbutyramides as acetyl-and butyrylcholinesterase inhibitors. Preparation, anticholinesterase activity, docking study, and 3D structure–activity relationship based on molecular interaction fields, Bioorg. Med. Chem., 2010, vol. 18, no. 3, pp. 1181–1193. https://doi.org/10.1016/j.bmc.2009.12.042
Teng, C.M., Hsueh, C.M., Chang, Y.L., Ko, F.N., Lee, S.S., and Liu, K.C.S., Antiplatelet effects of some aporphine and phenanthrene alkaloids in rabbits and man, J. Pharm. Pharmacol., 1997, vol. 49. N7, pp. 706–711. https://doi.org/10.1111/j.2042-7158.1997.tb06096.x
Huang, C.H., Huang, W.J., Wang, S.J., Wu, P.H., and Wu, W.B., Litebamine, a phenanthrene alkaloid from the wood of Litsea cubeba, inhibits rat smooth muscle cell adhesion and migration on collagen, Eur. J. Pharmacol., 2008, vol. 596, nos. 1–3, pp. 25–31. https://doi.org/10.1016/j.ejphar.2008.08.013
Chiou, C.M., Kang, J.J., and Lee, S.S., Litebamine N-homologues: Preparation and anti-acetylcholinesterase activity, J. Nat. Products., 1998, vol. 61, no. 1, pp. 46–50. https://doi.org/10.1021/np970298f
Zubenko, A.A., Morkovnik, A.S., Divaeva, L.N., Kartsev, V.G., Kuzmina, L.G., Borodkin, G.S., and Klimenko, A.I., Recyclization of glaucine as a new route to litebamine derivatives, Mendeleev Commun., 2018, vol. 28, no. 1, pp. 58–60. https://doi.org/10.1016/j.mencom.2018.01.019
Vetrova, E.V., Kurbatov, S.V., Borisenko, S.N., Lekar, A.V., Khizrieva, S.S., Borisenko, N.I., and Minkin, V.I., Synthesis of phenanthrene alkaloids from herbal aporphine alkaloids in subcritical water using synthesis of seco-glaucine as an example, Russ. J. Phys. Chem. B, 2017, vol. 11, no. 8, pp. 1255–1259. https://doi.org/10.1134/S1990793117080140
Borisenko, S.N., Lekar, A.V., Maksimenko, E.V., Khizrieva, S.S., Borisenko, N.I., and Minkin, V.I., Synthesis of phenanthrene alkaloids in subcritical water using secoboldine as an example, Chem. Natl. Compd., 2020, vol. 56, no. 1, pp. 183–184. https://doi.org/10.1007/s10600-020-02981-9
Vetrova, E.V., Kurbatov, S.V., Borisenko, S.N., Lekar’, A.V., Khizrieva, S.S., Borisenko, N.I., and Minkin, V.I., Synthesis of seco-glaucine as a model study for synthesis of phenanthridone alkaloids from herbal aporphine alkaloids, Sverkhkrit. Flyuidy: Teor. Prakt., 2017, vol. 12, no. 2, pp. 19–25.
Lekar’, A.V., Maksimenko, E.V., Borisenko, S.N., Khizrieva, S.S., Borisenko, N.I., and Minkin, V.I., ‘One-pot’ technique for transformation of the aporphine alkaloid boldine to phenanthrene seco-boldine by subcritical water, Sverkhkrit. Flyuidy: Teor. Prakt., 2019, vol. 14, no. 4, pp. 34–41. https://doi.org/10.34984/SCFTP.2019.14.4.005
Turmukhambetov, A.Zh., Mukusheva, G.K., Seidakhmetova, R.B., Shul’ts, E.E., Shakirov, M.M., Bagryanskaya, I.Yu., Gatilov, Yu.V., and Adekenov, S.M., Synthesis and antimicrobial activity of quaternary salts of the alkaloid glaucine, Pharm. Chem. J., 2009, vol. 43, no. 5, pp. 255–257. https://doi.org/10.30906/0023-1134-2009-43-5-24-27
Spasova, M., Philipov, S., and Milkova, T., Amino acid derivatives of aporphinic alkaloid glaucine and their antioxidant activity, in Peptides for Youth, New York: Springer, 2009, pp. 267–268. https://doi.org/10.1007/978-0-387-73657-0_120
Estelles, R., Milian, L., Nabah, Y.N.A., Mateo, T., Cerdá-Nicolás, M., Losada, M., Ivorra, M.D., Issekutz, A.C., Cortijo, J., Morcillo, E.J., Blázquez, M.A., and Sanz, M.-J., Effect of boldine, secoboldine, and boldine methine on angiotensin II-induced neurtrophil recruitment in vivo, J. Leukocyte Biol., 2005, vol. 78, no. 3, pp. 696–704. https://doi.org/10.1189/jlb.0105048
Hostalkova, A., Siatka, T., Chlebek, J., Opletal, L., Drasar, P., and Cahlikova, L., Boldine alkaloids and prospects of their utilization, Chem. Listy, 2015, vol. 109, no. 11, pp. 846–855.
Milian, L., Estelles, R., Abarca, B., Ballesteros, R., Sanz, M.J., and Blázquez, M.A., Reactive oxygen species (ROS) generation inhibited by aporphine and phenanthrene alkaloids semi-synthesized from natural boldine, Chem. Pharm. Bull., 2004, vol. 52, no. 6, pp. 696–699. https://doi.org/10.1248/cpb.52.696
Kostelnik, A. and Pohanka, M., Inhibition of acetylcholinesterase and butyrylcholinesterase by a plant secondary metabolite boldine, BioMed Res. Int., 2018, vol. 2018, p. 9634349. https://doi.org/10.1155/2018/9634349
Hung, T.M., Thuong, P.T., Nhan, N.T., Mai, N.T.T., Quan, T.L., Choi, J.S., Woo, M.H., Min, B.S., and Bae, K., Cholinesterase inhibitory activities of alkaloids from Corydalis tuber, Nat. Product Sci., 2011, vol. 17, no. 2, pp. 108–112.
Mollataghi, A., Coudiere, E., Hadi, A.H.A., Mukhtar, M.R., Awang, K., Litaudon, M., and Ata, A., Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species, Fitoterapia, 2012, vol. 83, no. 2, pp. 298–302. https://doi.org/10.1016/j.fitote.2011.11.009
de Lima, N.M.R., Ferreira, E.D.O., Fernandes, M.Y.S., Lima, F.A.V., Neves, K.R.T., do Carmo, M.R.S., and de An-drade, G.M., Neuroinflammatory response to experimental stroke is inhibited by boldine, Behav. Pharmacol., 2017, vol. 28, no. 2, pp. 223–237. https://doi.org/10.1097/FBP.0000000000000265
Yi, C., Ezan, P., Fernandez, P., Schmitt, J., Saez, J.C., Giaume, C., and Koulakoff, A., Inhibition of glial hemichannels by boldine treatment reduces neuronal suffering in a murine model of Alzheimer’s disease, Glia, 2017, vol. 65, no. 10, pp. 1607–1625. https://doi.org/10.1002/glia.23182
Cassels, B.K., Fuentes-Barros, G., and Castro-Saavedra S., Boldo, its secondary metabolites and their derivative, Curr. Tradit. Med., 2019, vol. 5, no. 1, pp. 31–65. https://doi.org/10.2174/2215083804666181113112928
Sharma, O.P. and Bhat, T.K., DPPH antioxidant assay revisited, Food Chem., 2009, vol. 113, no. 4, pp. 1202–1205. https://doi.org/10.1016/j.foodchem.2008.08.008
Khasanov, V.V., Ryzhova, G.L., and Mal’tseva, E.V., Methods for the study of antioxidants, Khim. Rastit. Syr’ya, 2004, no. 3, pp. 63–75.
Vetrova, E.V., Borisenko, N.I., Khizrieva, S.S., and Bugaeva, A.F., The study of antioxidant activity of the aporphine alkaloid of glaucine and the phenanthrene alkaloid of seco-glaucine obtained in subcritical water, Khim. Rastit. Syr’ya, 2017, no. 1, pp. 85–91. https://doi.org/10.14258/jcprm.2017011383
Zavilgelsky, G.B., Kotova, V.Y., and Manukhov, I.V., Action of 1,1-dimethylhydrazine on bacterial cells is determined by hydrogen peroxide, Mutat. Res. Genet. Toxicol. Environ. Mutagen., 2007, vol. 634, nos. 1–2, pp. 172–176. https://doi.org/10.1016/j.mrgentox.2007.07.012
Chistyakov, V.A., Prazdnova, E.V., Gutnikova, L.V., Sazykina, M.A., and Sazykin, I.S., Superoxide scavenging activity of plastoquinone derivative 10-(6'-plastoquinonyl) decyltriphenylphosphonium (SKQ1), Biochemistry (Moscow), 2012, vol. 77, no. 7, pp. 776–778
Ellman, G.L., Courtney, K.D., Andres, V., Jr., and Featherstone, R.M., A new and rapid colorimetric determination of acetylcholinesterase activity, Biochem. Pharmacol., 1961, vol. 7, no. 2, pp. 88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Omar, S.H., Scott, C.J., Hamlin, A.S., and Obied, H.K., Biophenols: Enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.), Fitoterapia, 2018, vol. 128, pp. 118–129. https://doi.org/10.1016/j.fitote.2018.05.011
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation (State assignment in the field of scientific activity, Southern Federal University, 2020, no. BAЗ0110/20-3-09IH) and by the Russian Foundation for Basic Research (project no. 19-33-90211-Aspiranty).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Makeeva
Rights and permissions
About this article
Cite this article
Khizrieva, S.S., Borisenko, S.N., Maksimenko, E.V. et al. Antioxidant Properties and Effects of Aporphine Alkaloids and Their Phenanthrene Seco-Isomers on Acetylcholinesterase Activity. Russ J Bioorg Chem 48, 1433–1440 (2022). https://doi.org/10.1134/S106816202207010X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S106816202207010X