Abstract
This study represents the first report on the embryological characteristics of triploid male-sterile dandelion Taraxacum belorussicum (section Palustria) from Poland. While this taxon is considered to be a male-sterile species, we found that the investigated individuals produced pollen. Irregular tetrads, triads and diads with microspores of unequal size were observed in the pollen loculi as a result of disturbed meiotic division, while anthers’ tapetum did not show structural disorders. Possible reasons for the plasticity in the expression of male sterility, as well as the role of pollen in apomicts, are discussed. Flowers of the examined individuals contained well-developed nectaries. The course of embryological processes in the ovules indicated an apomictic mode of reproduction in T. belorussicum. We observed meiotic diplospory of the Taraxacum type, in which first meiotic division starts but results in nuclear restitution, while undisturbed second meiotic division gives rise to a dyad of unreduced megaspores (diplodyad). After three mitotic divisions of the chalazal megaspore, a seven-celled unreduced female gametophyte developed. The features of ovule anatomy and characteristics of a mature female gametophyte corresponded to these described in sexually reproducing dandelions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taraxacum Wigg. (dandelion) belonging to the subfamily Cichorioideae of the Asteraceae is a large cosmopolitan genus comprising approximately 3000 recognized species grouped into about 60 sections (Battjes et al. 1992; Kirschner et al. 2015). It represents an agamic complex, in which sexual diploids or tetraploids coexist with apomictic polyploids (Kirschner and Štěpánek 1996). In the sexual pathway of reproduction, regular meiosis and double fertilization are indispensable for seeds formation, whereas these processes are omitted during seeds’ production in agamospermous dandelions, resulting in offspring that are genetically identical to the mother plant. For this reason, the genus Taraxacum is often used as a model system not only in research on molecular mechanisms underlying apomixis (Vijverberg et al. 2010 and references therein), but also for comparative embryological analysis of the sexual and apomictic reproductive modes (Małecka 1971a, 1973; Van Baarlen et al. 2000, 2002; Musiał and Kościńska-Pająk 2013; Musiał et al. 2013b, 2015; Płachno et al. 2014, 2015).
It is well known that the seeds of apomictic dandelions develop completely independent of the pollen, as a result of meiotic diplospory, parthenogenesis, and autonomous endosperm formation (Gustafsson 1946; Richards 1973). Furthermore, crossing experiments and genetic analyses revealed that in Taraxacum these basic elements of apomictic developmental pathway are controlled by three dominant loci, and that diplospory, as well as parthenogenesis, are inherited independently, while the inheritance of autonomous endosperm formation is still not fully settled (Van Dijk et al. 1999, 2003; Van Dijk and Bakx-Schotmann 2004; Vijverberg et al. 2004, 2010).
Despite highly advanced genetic and molecular research on dandelions’ reproduction, the documentation of microscopic observations of megaspores formation, female gametophyte development and its structure, embryogenesis and endosperm origination is mainly presented in the form of drawings in the papers that appeared many years ago, e.g., Gustafsson (1946), Fagerlind (1947), Battaglia (1948), and Małecka (1965, 1982). At the beginning of the twenty-first century, a clearing tissue technique has been applied to the examination of dandelions’ generative structures with the use of differential interference contrast microscopy (Van Baarlen et al. 2000, 2002; Van Dijk et al. 2003). This method gives a possibility to investigate in toto non-stained flowers, anthers, ovaries or dissected ovules and is less time-consuming, compared to other standard techniques used in embryological studies, e.g., technique of paraffin or Technovit sections (Jensen 1962; O’Brien and McCully 1981). It also allows for a detailed cyto-embryological analysis of both microsporogenesis and megasporogenesis in Taraxacum species (Van Baarlen et al. 2000, 2002; Musiał et al. 2015).
In the present study, we applied this method to the embryological investigations of male-sterile Taraxacum belorussicum Val.N.Tikhom. belonging to Taraxacum sect. Palustria. This section comprises above 130 species growing mainly in Europe and, just like the other Taraxacum sections, is dominated by polyploid taxa, whereas diploids are represented only by two very rare Mediterranean species: T. raii Gray and T. tenuifolium H.Koidz., respectively (Marciniuk et al. 2010a, b, 2012; Carlesi and Peruzzi 2012). Dandelions classified to this section are listed as infrequent, poorly known and threatened with extinction because of the degradation of their natural habitats by disastrous human activity (Van Soest 1965; Kula et al. 2013; Musiał et al. 2013a).
Taraxacum belorussicum is a species of very poorly recognized distribution and uncertain taxonomic position. This triploid (2n = 3x = 24) dandelion is known only from the Minsk region in Belarus (locus classicus) and a single locality in the north-eastern Poland, and is morphologically greatly similar to other triploid species from the section Palustria, namely to T. dentatum Kirschner et Štěpánek (Tikhomirov 2003; Marciniuk et al. 2010b; Kula et al. 2013). The presence of pollen in T. dentatum and its absence in T. belorussicum are the only feature clearly differentiating both taxa. Actually, it cannot be ruled out that specimens of T. belorussicum collected in Poland represent an unknown male-sterile line of T. dentatum (J. Štěpánek personal comm.). Hence, the taxonomic differentiation of both species requires not only further studies including reproductive traits recognition, but also analyses at the molecular level.
Disparity observed between taxonomic data and our preliminary analysis with respect to pollen production in the individuals of this species prompted us to undertake this detailed embryological research of male-sterile T. belorussicum. Here, we document for the first time the developmental processes occurring in anthers as well as in ovaries and ovules of Polish representatives of male-sterile T. belorussicum using clearing tissue technique and Nomarski contrast microscopy. We believe that the results of the present embryological studies may, in the future, prove to be useful in resolving the taxonomic position of male-sterile T. belorussicum and male-fertile T. dentatum.
Materials and methods
Inflorescences collected from seven randomly selected plants within Polish natural population of T. belorussicum in Mścichy, a weir in the Biebrza National Park (53°25′43″N, 22°29′4″E) served as the material for the research. A voucher specimen was deposited at the herbarium of the Jagiellonian University in Kraków (KRA). The whole capitula were fixed in a modified Carnoy’s fluid (96 % ethanol + glacial acetic acid in a volume ratio 3:1). After 24 h the material was transferred to 70 % ethanol and stored at 4 °C. Embryological examination was performed on at least three capitula from each plant, and about 280 individual florets at various developmental stages were analyzed. Whole florets, ovaries, and ovules were then isolated and dehydrated for 1 h in ethanol series: 70, 80 (one change) and 100 % (three changes). Dehydrated samples were cleared in methyl salicylate (Sigma-Aldrich) according to a procedure described by Musiał et al. (2015). Cleared material was placed in a drop of pure methyl salicylate on a Rajs’ slides (Herr 2000) and examined using a Nikon Eclipse 80i microscope fitted with Nomarski’s Interference Contrast (DIC optics).
Results
The anthers
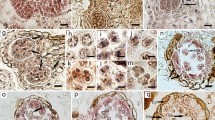
The inflorescence of T. belorussicum consists of only ligulate florets, which is typical of the Cichorioideae. Florets are hermaphroditic and have five normally developed stamens with connate anthers forming a tube around the pistil style. In the analysed material, archesporial cells arranged in a single row and surrounded by a layer of flattened uninucleate tapetal cells were recorded in the pollen loculi of only a few florets, whereas the course of microsporogenesis was not observed. In the majority of the examined flowers, anthers were already at the post-meiotic stages. However, the presence of very irregular tetrads indicated that male meiosis was strongly disturbed. Usually, as a result of meiotic division, tetrads with unequal size of microspores, polyads, and numerous dyads were formed (Fig. 1a–c). At this developmental stage, the microspores within tetrads often began to degenerate (Fig. 1d), and then only the remnants of degenerated cells enclosed in a common callose wall could be observed (Fig. 1e). Nevertheless, after the dissolution of callose walls in the tetrads, some of the anthers were filled with free microspores, surrounded by a sporoderm, in which a large nucleus with conspicuous nucleolus was situated centrally, but the cytoplasm of these microspores showed abnormal vacuolation (Fig. 1f). Frequently, the protoplasts of the free microspores became highly shrunk and separated from the sporoderm (Fig. 1g). In spite of the observed abnormalities, regular microspores, containing a dense cytoplasm around the centrally situated nucleus, as well as bicellular pollen grains, were present in a number of examined anthers (Fig. 1h, i). As a rule, anthers’ tapetum did not show structural disorders. The tapetal cells were initially uninucleate, and in the subsequent developmental stages they became multinucleate, as a result of mitosis with inhibited cytokinesis (Fig. 1a–c). Along with the dissolution of callose in the tetrads, a partial breakdown of the cell walls usually occurred in the tapetal cells and ameboid tapetum was formed (Fig. 1f). However, in some anthers, the tapetum cells showed structural abnormalities connected with an unusual vacuolation of their cytoplasm (Fig. 1d, e). At the stage of bicellular pollen grains, the tapetal layer was already absent or only its remnants were visible in the anthers (Fig. 1i).
Developmental events in anthers of Taraxacum belorussicum. a–c Fragments of anthers containing tetrads with unequal size of microspores, polyads, and dyads surrounded by proper tapetal layer. d, e Microspores degeneration at the tetrad stage, arrows indicate degenerated cells; note unusual vacuolation of tapetal cells. f Microspores with centrally located nucleus and abnormal vacuolation of cytoplasm (arrowheads). g Degenerated microspores with strongly shrunk protoplasts surrounded by sporoderm. h Viable young microspores. i Bicellular pollen grains; arrows indicate sporoderm. t tapetum. Scale bars a–g, i 10 μm; h 20 μm
Ovary and ovule structure
Taraxacum belorussicum has a bicarpellate gynoecium with an inferior and unilocular ovary, which contains a single ovule on the basal placenta (Fig. 2a, b). The outer surface of a young ovary was initially entirely smooth (Fig. 2a), but in older flowers, distinctive protuberances emerged on the surface in the apical region of the ovary (Fig. 2b, d). These outgrowths were gradually increasing in size and finally developed in spikes, which formed a specific sculpture on the surface of the achenes. In young flowers, nectaries are visible above the ovary as small multicellular bulges at the base of the style (Fig. 2c), and then they form bigger cone-shape structures (Fig. 2d). Mature ovules are anatropous, tenuinucelate and unitegmic (Fig. 2b, e). The integument is massive and initially has a homogeneous structure (Fig. 2a) but subsequently, it shows a distinct zonal differentiation (Fig. 2b, e). The inner epidermis of the integument differentiated into an integumentary tapetum (endothelium) directly surrounding the female gametophyte (Fig. 2e), and around the endothelial layer a characteristic zone of thick-walled cells is visible (Fig. 2b, e). In a fully developed ovule, at the base of the funiculus, an obturator is visible in the form of a group of elongated cells protruding towards the micropylar canal (Fig. 1e, f).
Ovary and ovule of Taraxacum belorussicum. a Inferior and unilocular ovary at early developmental stage. b Older ovary with anatropous, unitegmic and tenuinucelate ovule. Nectaries at early (c) and older (d) developmental stage. e Dissected ovule with visible zone of thick-walled integumentary cells (dashed line) surrounding the embryo sac (arrowhead); micropylar canal is framed. f Magnification of the part framed in e shows elongated obturator cells (black arrowhead) in micropylar canal. ch chalazal pole, f funicle, int integument, m micropylar canal, n nectary, black arrows outgrowths of the ovary wall, layer of thick-walled integumentary cells; star ovary chamber, white arrow lack of outgrowths on the outer surface of ovary wall. Scale bars a, b, d, e 100 μm; c 10 μm, f 20 μm
Megasporogenesis
In a very young ovule, a single archesporial cell, which differentiated just below the nucellus epidermis, elongates in the micropylar-chalazal axis and functions directly as the megaspore mother cell (MMC). Megasporogenesis in T. belorussicum, like in other apomictic dandelions, proceeds according to the meiotic diplospory of the Taraxacum type, which involves first division restitution (FDR) and normal second meiotic division, resulting in the dyad of unreduced megaspores. In the early prophase I, the MMC had a dense cytoplasm and centrally located large nucleus, in which a prominent nucleolus and strands of condensed chromosomes were visible (Fig. 3a). The nucleolus initially occupied a central position in the nucleus, but then it gradually moved to the periphery of the nucleus, where it was observed during diakinesis (Fig. 3a, b). The pairing of chromosomes was incomplete and instead of bivalents, mainly univalents were formed. A reduced pairing was followed by a strongly disturbed metaphase I during which the bivalents and univalents were usually scattered along the spindle and only sometimes grouped in the almost equatorial plate (Fig. 3c–e). Then they were surrounded by the nuclear envelope and a restitution nucleus of ellipsoidal shape formed as a result of this semi-heterotypic division (Fig. 3f). The second meiotic division proceeded without any disturbances and led to the formation of a diplodyad (Fig. 4a–e). In the early telophase II, a system of microtubular bundles of the phragmoplast was clearly visible between the two regular daughter nuclei (Fig. 4a). Then, the cell plate began to form in the equatorial plane of the phragmoplast (Fig. 4b–d), and the growing cell plate finally reached the plasma membrane of MMC completely separating the protoplasts of two daughter cells within the diplodyad (Fig. 4e). In the ovules of T. belorussicum, the differentiation of integumentary tapetum initiated at the MMC stage. As early as during metaphase I, the beginnings of a gradual degeneration of nucellus cells were seen, especially around the chalazal pole of the megasporocyte, and a layer of integumentary tapetum adjacent to the MMC was present (Fig. 3c–f).
First division restitution in Taraxacum belorussicum megaspore mother cell. a, b Prophase I. c–e Disturbed metaphase I. f Ellipsoid restitution nucleus (arrowhead) formed after disturbed first meiotic division. ch chalazal pole, it integumentary tapetum, m micropylar canal, arrows show epidermis of nucellus. Scale bars 10 μm
Diplodyad formation in Taraxacum belorussicum. a Telophase II in MMC, arrowhead indicates phragmoplst. b–d Cytokinesis and progressive formation of cell plate (arrows). e Young diplodyad; arrow indicates thin cell wall between megaspores. ch chalazal pole, it integumentary tapetum, m micropylar canal. Scale bars 10 μm
Female gametophyte formation and megagametogenesis
Newly formed megaspores are of the same size and have a dense cytoplasm (Fig. 4d, e) but later, the chalazal one enlarges and develops into a functional megaspore, while the micropylar cell of diplodyad gradually degenerates (Fig. 5a). The functional megaspore contains a large nucleus located in the central region of the cell and cytoplasm with clearly visible vacuoles, especially at the chalazal pole (Fig. 5a). Unreduced female gametophyte of T. belorussicum develops from the functional megaspore by three successive mitotic nuclear divisions. The first mitosis led to the formation of two-nucleate embryo sac, in which two daughter nuclei migrated to the opposite poles of the syncytium, and a prominent vacuole appeared between them (Fig. 5b, c). Fairly large vacuoles were also found below the nucleus at the chalazal pole of syncytium, while the cytoplasm above the micropylar nucleus showed no vacuolation (Fig. 5b, c). With the development of the female gametophyte, degeneration of the surrounding nucellar cells was evident, and the embryo sac was directly adjacent to the well-developed layer of the integumentary tapetum (Fig. 5c). Two subsequent mitoses gave rise to an eight-nucleate embryo sac, which then underwent cellularisation. Then, a fully developed female gametophyte of T. belorussicum was a seven-celled structure organized according to the embryo sac of the Polygonum type. It contained an egg apparatus at the micropylar pole, three antipodal cells at the chalazal pole, and a highly vacuolated central cell in the middle part of the embryo sac (Fig. 6a–c). The egg apparatus consisted of three pear-shaped cells, a larger egg cell and two smaller synergids. Individual cells of the egg apparatus, as well as the central cell, showed a typical polarity at the sub-cellular level, manifested by polar distribution of the cytoplasm and a specific position of the nuclei. Namely, the chalazal parts of the synergids were strongly vacuolated, and most of the synergids’ cytoplasm was situated in the micropylar regions of the cells (Fig. 6a, b, d). The micropylar part of the egg cell, however, is occupied by a large vacuole, and the nucleus surrounded by a dense cytoplasm is located in the chalazal part of the cell (Fig. 6a–c). In a highly vacuolated central cell, however, the cytoplasm is largely accumulated at its micropylar region. The polar nuclei or the secondary nucleus, formed after the fusion of the polar nuclei, were situated very close to the nucleus of the egg cell (Fig. 6a–c). In older embryo sacs, the synergid cells were distinguished by a slightly different shape. Their micropylar tops became elongated and penetrated into the micropylar canal where, they overlapped in a hook-like manner, moreover, distinctly thickened walls between the synergids at the micropylar pole could be observed (Fig. 6d, e). Antipodal cells persisted for a long time and were visible in the mature female gametophytes. At this stage, three clearly visible antipodal cells show no signs of degeneration. Individual antipodal cells are mononucleate and contain dense cytoplasm (Fig. 6a–c). In the course of the present investigations, an occasional occurrence of sterile ovules was observed. In such ovules, gradual shrinkage of the cells of the female gametophyte take place (Fig. 6f), and finally the embryo sac collapsed completely (Fig. 6g). However, this process is not accompanied by disintegration of the integumentary tapetum (Fig. 6f, g).
Early stages of megagametogenesis in Taraxacum belorussicum. a One-nucleate embryo sac; arrow points to degenerating micropylar megaspore. b, c Two-nucleate embryo sac; arrowheads indicate degenerated cells of nucellus. ch chalazal pole, it integumentary tapetum, m micropylar canal, v vacuole. Scale bars 10 μm
Structure of female gametophyte of Taraxacum belorussicum. a Seven-celled embryo sac with polar nuclei in central cell. b, c Mature embryo sac; dashed line indicates secondary nucleus in central cell. d, e Micropylar part of mature embryo sac; arrowhead points to nucleus in synergid cell, note hooked micropylar tops of synergids (arrows). f, g Progressive degeneration of embryo sac; arrow points to completely collapsed embryo sac. a antipodal cell, cc central cell, ec egg cell, it integumentary tapetum, s synergid, v vacuole. Scale bars 10 μm
Discussion
The majority of apomictic plants is pseudogamous (Nogler 1984; Mogie 1992; Koltunow and Grossniklaus 2003; Van Dijk 2003, 2009). They are capable of developing embryos independent of fertilization of the egg cell; however, pollination is necessary for the fertilization of the central cell and the initiation of endosperm development essential for viable seed formation. Only autonomous apomicts are entirely independent of pollination for the embryo and endosperm development. Autonomous apomixis is particularly frequent within the Asteraceae and occurs mainly among diplosporous species, like Taraxacum and Chondrilla taxa (Nogler1984; Asker and Jerling 1992; Van Dijk 2003; Kościńska-Pająk and Bednara 2006; Noyes 2007). Although in autonomous apomicts, the endosperm, similar to the embryo, develops autonomously, most of them maintain the male function and continue to produce functional pollen. This is commonly observed within the Taraxacum genus (Małecka 1971a, 1973; Van Baarlen et al. 2000; Marciniuk et al. 2010a; Musiał et al. 2013a). Maynard Smith (1978) suggested that apomicts producing pollen are phylogenetically relatively young to have accumulated the necessary mutations that cause male sterility. However, male sterility has been found in both diploid sexual and apomictic dandelions representing numerous sections (Van Soest 1965; Richards 1970; Małecka 1971b; Van der Hulst et al. 2004; Meirmans et al. 2006). Pollen production is linked to a significant reproductive cost, thus, the male sterility in apomicts can be considered as a reallocation of resources from male function to increase female fitness. This is confirmed by observations of Meirmans et al. (2006), who discovered that male-sterile apomictic dandelions produce more capitula per plant and thereby more seeds than pollen producing ones.
The actual male function in autonomous apomicts is still not fully recognized, but the role of pollen in natural apomicts is emphasized for an indirect transmission of apomixis genes to sexuals and the spread of apomixis by new asexual lineages formation (Van Dijk 2003; Verduijn et al. 2004; Mártonfiová 2006; Meirmans et al. 2006; Whitton et al. 2008). Different possible pathways of gene flow among sexual and apomictic Taraxacum species have been suggested and confirmed by experimental crosses: (1) BII hybrids formation (hybrids resulting from fertilization of sexuals by apomicts pollen), (2) BIII hybrids formation (hybrids resulting from fertilization of unreduced egg cells), (3) facultative apomixis (occasional formation of reduced megagametophytes that require feritilization) (Małecka 1973; Mártonfiová 2006 and references therein).
In apomictic dandelions, male meiosis is usually reductional but, especially in triploids, it is strongly disturbed and leads to the production of irregular pollen, from haploid to unreduced triploid pollen grains. A detailed analysis of early stages of microsporogenesis in the triploids T. palustre Dalhst. (Małecka 1965), T. kok-saghyz Rodin (Małecka 1971a), T. alatum H.Lindb. (Małecka 1982), and T. officinale F.H.Wigg. (Van Baarlen et al. 2000) revealed significant reduction of chromosome pairing and deficient chiasma formation at prophase I, resulting in high numbers of univalents, relatively low number of bivalents, and the formation of trivalents. In these dandelion species, abnormalities were also observed in the later stages of pollen meiosis. Thus, an aberrant meiotic cycle causes the formation of unbalanced microspores of variable chromosome numbers, and in consequence, the pollen of triploid dandelions is characterized by a great variability of size and viability, as it was demonstrated in many species, including dandelions from the section Palustria (Małecka 1965, 1971a, 1973; Marciniuk et al. 2010a; Musiał et al. 2013a). Similarly, in the examined anthers of T. belorussicum, the presence of irregular tetrads of microspores indicates that the course of microsporogenesis was abnormal. Within the Taraxacum genus, morphological diversity of pollen is related to the mode of reproduction, i.e., the pollen of sexual diploids is very regular, facultative apomicts produce rather regular pollen of low size variability, while the pollen of obligatory apomicts is very irregular and is characterized by high size variability (Małecka 1973; Sterk et al. 1982; Marciniuk et al. 2010a). In general, the pollen of apomictic Taraxacum species is largely sterile, nevertheless, some pollen grains are viable and may be capable of reproductive processes. For example, in male-fertile triploid dandelion T. udum Jord. (sect. Palustria), the heterogeneous-sized pollen was of relatively poor quality, however, almost 41 % of the pollen grains contained cytoplasm, which stained red using the acetocarmine test (Musiał et al. 2013a).
On the basis of observations of the anther wall structure in male-sterile agamospermous T. balticum Dahlst. (sect. Palustria) and T. silesiacum Dahlst. & G.E.Haglund (=T. parnassicum Dahlst.) (sect. Erythrosperma), Małecka (1971b) suggested for the first time the occurrence of cytoplasmic male sterility in dandelions. In further research, nuclear-cytoplasmic male sterility was found in sexually reproducing dandelions from two French populations (Van der Hulst et al. 2004). However, in male-sterile individuals of triploid dandelions from T. section Ruderalia, the results of crosses as well as microsatellites and the chloroplast haplotyping indicated that male sterility in apomictic dandelions has nuclear basis (Meirmans et al. 2006).
In male-sterile T. balticum and T. silesiacum, premature abortion of the tapetal layer in the anther wall was recorded already at the premeiotic stage, and this was the cause of malnutrition of the meiocytes and their early degeneration (Małecka 1971b). Such abnormal behaviour of the tapetum is frequently one of the first visible signs of cytoplasmic male sterility in plants (Schnable and Wise 1998; Sakata and Higashitani 2008). In the anthers of the investigated individuals of T. belorussicum we have not observed a premature breakdown of the tapetal layer, however, in some anthers, the cells of tapetum showed abnormal vacuolation. The formation of large vacuoles in the cytoplasm of tapetal cells is also an initial manifestation of their degeneration, and it has been reported in male-sterile Petunia hybrida as well as in many other plants demonstrating male sterility, for example in Zea mays, Capsicum annuum, Sorghum, Cajanus cajan, Glycine max (Bino 1985 and references therein).
Although total lack of pollen production is regarded as an important feature of diagnostic value in the taxonomy of dandelions (Marciniuk 2012), it has been found that individuals can exhibit plasticity in the expression of male sterility. For example, some male-sterile dandelion plants formed male-sterile, partially male-sterile or fully male-fertile capitula (Meirmans et al. 2006). Variation in pollen production within the same capitulum, or even within the same flower, was also stated in triploid Hieracium villosum (Urbanska 1991) and H. alpinum (Mráz et al. 2009). Moreover, a different population of male-sterile apomictic species can often exhibit plasticity in the production of pollen, as it was recorded in two Scandinavian populations of Antennaria alpina (Urbanska 1994) as well as in different European populations of Hieracium alpinum (Slade and Rich 2007; Mráz et al. 2009). In addition, populations of some dandelion species may differ in this respect, for example, T. rubicundum Dahlst. usually produces pollen in the south of Europe, but is male-sterile in the north (Richards 1997). Intraspecific polymorphism in pollen production may be due to environmental factors, as it has been observed in the British taxa of Hieracium sect. Alpina (Slade and Rich 2007). It has been shown that high temperature is the main abiotic stress factor which causes male sterility in many plant species (reviewed by Sakata and Higashitani 2008). The effect of the environment on male sterility, especially the impact of day length and temperature, was reported in Cajanus cajan, where high temperatures and longer days maintained male-sterility, while shorter days and lower temperature induced male fertility (Saxena et al. 2010). Similarly, in some individuals of the male-sterile diploid dandelions, the production of pollen depended on the temperature (Van der Hulst et al. 2004). In the light of these findings, it can be assumed that the presence of pollen observed in the investigated individuals of male-sterile T. belorussicum from Poland is a sign of such intraspecific variation in pollen production, which may be affected by environmental factors. Undoubtedly, it would be advisable to carry out comparative embryological studies within the Belarusian population of this species, together with an analysis of molecular markers of male sterility. In the future, additional embryological and molecular comparisons are also needed to determine phylogenetic relationships of male-sterile T. belorussicum and pollen producing T. dentatum.
In the examined flowers of T. belorussicum, we found well-developed nectaries, which we have also observed in another male-sterile dandelion T. atricapillum Sonck from section Borea (unpublished data). These observations indicate that even male-sterile dandelions are likely to be capable of producing nectar. This is another example, alongside the formation of functionless yellow petals, that autonomous apomicts usually maintain pollinator attraction features.
Application of tissue-clearing technique in the present embryological studies also allowed us to recognize the reproductive processes in the ovules of T. belorussicum. We have observed that in this triploid species, as in other apomictic dandelions, reproductive development involves meiotic diplospory leading to the formation of two unreduced megaspores. The chalazal one develops by three mitotic divisions into seven-celled female gametophyte of the organization corresponding to the Polygonum type. It should be stressed that in apomictic dandelions, in contrast to the reductional pollen meiosis, mostly asynaptic female meiosis is restitutional to avoid the formation of unbalanced megaspores and sterility (Van Baarlen et al. 2000 and references therein). Only in amphimictic dandelions, like in most angiosperms, female meiosis follows the regular course and produces a linear array of four reduced megaspores, one of which develops into seven-celled female gametophyte, while three cells degenerate. Regular meiotic division and linear tetrads of megaspores has been documented in the ovules of diploid dandelions, for example, in T. pieninicum Pawł. (Małecka 1961), diploid sexuals of T. officinale (Van Baarlen et al. 2000, 2002), and most recently in diploid T. linearisquameum Soest (Musiał et al. 2015).
The most recent ultrastructural comparative studies showed no essential differences in the ovule anatomy, in the structure of micropyle canal and also synergid cells between sexual and apomictic dandelions (Musiał et al. 2013b; Płachno et al. 2014, 2015). Results of these studies, as well as the present observations of the ovule anatomy and female gametophyte structure in T. belorusscium, clearly indicate that, although in apomictic dandelions the development of both embryo and endosperm is autonomous, their ovules and unreduced female gametophytes permanently retain features that are associated with sexual reproduction. This can be explained by the fact that apomictic Taraxacum species are considered to be relatively recent in origin and hence the functionless characters are still maintained (Richards 1973; Maynard Smith 1978; Van Dijk 2003). On the other hand, Płachno et al. (2015) pointed out that the presence of vital micropylar transmitting tissue and synergid cells with a filiform apparatus makes no barrier for pollen tube to reach the ovule and female gametophyte, thus fertilization of an unreduced egg cell of apomicts is likely, which may be essential in the light of possible hybridization among sexuals and apomicts and the creation of new apomictic lineages, advantageous from an evolutionary point of view.
In conclusion, although triploid T. belorussicum is considered to be a male-sterile dandelion, we showed that the anthers of specimens covered by this study contained pollen grains. Thus, we can conclude that a total lack of pollen is not stable and unambiguous trait in male sterile dandelions and therefore it cannot be regarded as a fully reliable feature of diagnostic value and taxonomic importance. The course of developmental processes in the ovules indicated an apomictic mode of reproduction. Despite this, flowers of T. belorussicum maintain pollinator attraction as evidenced by the presence of well-developed nectaries. The main results related to the female reproductive pathway of this species can be summarized in the following way: (1) inferior and unilocular ovary contains one anatropous, unitegmic and tenuinucelate ovule; (2) megaspores are formed by meiotic diplospory, (3) the chalazal cell of diplodyad is the functional megaspore; (4) unreduced female gametophyte develops by three successive mitotic nuclear divisions; (5) organization of mature female gametophyte corresponds to the Polygonum type.
References
Asker SE, Jerling L (1992) Apomixis in plants. CRC Press, Boca Raton
Battaglia E (1948) Ricerche sulla parameiosi restitutionale nel genere Taraxacum. Caryologia 1:1–47
Battjes J, Menken SBJ, Den Nijs HCM (1992) Clonal diversity in some microspecies of Taraxacum sect. Palustria (Lindb. fil.) Dahlst. from Czechoslovakia. Bot Jahrb Syst Pflanzengesch Pflanzengeogr 114:315–328
Bino RJ (1985) Ultrastructural aspects of cytoplasmic male sterility in Petunia hybrida. Protoplasma 127:230–240. doi:10.1007/BF01276267
Carlesi V, Peruzzi L (2012) The genus Taraxacum (Asteraceae, Cichorieae) in Italy IV. Two new species of T. sect. Palustria. Willdenowia 42:191–197. doi:10.3372/wi.42.42204
Fagerlind F (1947) Makrosporogenese und Embryosackbildung bei agamospermischen Taraxacum-Biotypen. Svensk Bot Tidskr 41:365–390
Gustafsson Å (1946) Apomixis in higher plants. Part I. The mechanisms of apomixis. Lunds Univ Årsskr N F Avd 42:1–66
Herr JM Jr (2000) Clearing techniques for unusual studies in seed plant embryology. Bot Guidebooks 24:25–39
Jensen WA (1962) Botanical histochemistry. WH Freeman and Co., San Francisco
Kirschner J, Štěpánek J (1996) Modes of speciation and evolution of the sections in Taraxacum. Folia Geobot Phytotax 31:415–426. doi:10.1007/BF02815386
Kirschner J, Záveská Drábková L, Štěpánek J, Uhlemann I (2015) Towards a better understanding of the Taraxacum evolution (Compositae–Cichorieae) on the basis of nrDNA of sexually reproducing species. Pl Syst Evol 301:1135–1156. doi:10.1007/s00606-014-1139-0
Koltunow AM, Grossniklaus U (2003) Apomixis: a developmental perspective. Annual Rev Pl Biol 54:547–574. doi:10.1146/annurev.arplant.54.110901.160842
Kościńska-Pająk M, Bednara J (2006) Unusual microtubular cytoskeleton of apomictic embryo sac of Chondrilla juncea L. Protoplasma 227:87–93. doi:10.1007/s00709-006-0147-5
Kula A, Grabowska-Joachimiak A, Kasjaniuk M, Legutko J, Marciniuk P, Musiał K (2013) Chromosome numbers in 10 Taraxacum species from Poland. Acta Biol Cracov Ser Bot 55:153–157. doi:10.2478/abcsb-2013-0030
Małecka J (1961) Studies in the mode of reproduction of the diploid endemic species Taraxacum pieninicum Pawł. Acta Biol Cracov Ser Bot 4:25–46
Małecka J (1965) Embryological studies in Taraxacum palustre. Acta Biol Cracov Ser Bot 8:223–235
Małecka J (1971a) Cyto-taxonomical and embryological investigations on a natural hybrid between Taraxacum kok-saghyz Rodin and T. officinale Web. and their putative parent species. Acta Biol Cracov Ser Bot 14:179–197
Małecka J (1971b) Processes of degeneration in anthers’ tapetum of two male-sterile species of Taraxacum. Acta Biol Cracov Ser Bot 14:1–10
Małecka J (1973) Problems of the mode of reproduction in microspecies of Taraxacum section Palustria Dahlsted. Acta Biol Cracov Ser Bot 16:37–84
Małecka J (1982) Further embryological studies in the genus Taraxacum L. Acta Biol Cracov Ser Bot 24:143–157
Marciniuk J (2012) Taraxacum sect. Palustria in Poland. University of Natural Sciences and Humanities Press, Siedlce
Marciniuk J, Grabowska-Joachimiak A, Marciniuk P (2010a) Differentiation of the pollen size in five representatives of Taraxacum sect. Palustria. Biologia 65:954–957. doi:10.2478/s11756-010-0107-6
Marciniuk J, Rerak J, Grabowska-Joachimiak A, Jastrząb I, Musiał K, Joachimiak AJ (2010b) Chromosome numbers and stomatal cell length in Taraxacum sect. Palustria from Poland. Acta Biol Cracov Ser Bot 52:117–121. doi:10.2478/v10182-010-0015-7
Marciniuk P, Musiał K, Joachimiak AJ, Marciniuk J, Oklejewicz K, Wolanin M (2012) Taraxacum zajacii (Asteraceae), a new species from Poland. Ann Bot Fenn 49:387–390. doi:10.5735/085.049.0611
Mártonfiová L (2006) Possible pathways of the gene flow in Taraxacum sect. Ruderalia. Folia Geobot 41:183–201. doi:10.1007/BF02806478
Maynard Smith J (1978) The evolution of sex. Cambridge University Press, Cambridge
Meirmans PG, Den Nijs JCM, Van Tienderen PH (2006) Male sterility in triploid dandelions: asexual females vs asexual hermaphrodites. Heredity 96:45–52. doi:10.1038/sj.hdy.6800750
Mogie M (1992) The evolution of asexual reproduction in plants. Chapman and Hall, London
Mráz P, Chrtek J, Šingliarová B (2009) Geographical parthenogenesis, genome size variation and pollen production in the arctic-alpine species Hieracium alpinum. Bot Helv 119:41–51. doi:10.1007/s00035-009-0055-3
Musiał K, Kościńska-Pająk M (2013) Egg apparatus in sexual and apomictic species of Taraxacum: structural and immunocytochemical aspects of synergid cells. Acta Biol Cracov Ser Bot 55:107–113. doi:10.2478/abcsb-2013-00011
Musiał K, Górka P, Kościńska-Pająk M, Marciniuk P (2013a) Embryological studies in Taraxacum udum Jordan (sect. Palustria). Botany 91:614–620. doi:10.1139/cjb-2013-0022
Musiał K, Płachno BJ, Świątek P, Marciniuk J (2013b) Anatomy of ovary and ovule in dandelions (Taraxacum, Asteraceae). Protoplasma 250:715–722. doi:10.1007/s00709-012-0455-x
Musiał K, Kościńska-Pająk M, Antolec R, Joachimiak AJ (2015) Deposition of callose in young ovules of two Taraxacum species varying in the mode of reproduction. Protoplasma 252:135–144. doi:10.1007/s00709-014-0654-8
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of angiosperms. Springer, Berlin, pp 475–518
Noyes RD (2007) Apomixis in the Asteraceae: diamonds in the rough. Funct Pl Sci Biotechnol 1:207–222
O’Brien TP, McCully ME (1981) The study of plant structure: principles and selected methods. Termarcarphi Pty. Ltd., Melbourne
Płachno BJ, Musiał K, Świątek P, Tuleja M, Marciniuk J, Grabowska-Joachimiak A (2014) Synergids and filiform apparatus in the sexual and apomictic dandelions from section Palustria (Taraxacum, Asteraceae). Protoplasma 251:211–217. doi:10.1007/s00709-013-0539-2
Płachno BJ, Świątek P, Kozieradzka-Kiszkurno M, Majeský L, Marciniuk J, Stolarczyk P (2015) Are obligatory apomicts invested in the pollen tube transmitting tissue? Comparison of the micropyle ultrastructure between sexual and apomictic dandelions (Asteraceae, Lactuceae). Protoplasma 252:1325–1333. doi:10.1007/s00709-015-0765-x
Richards AJ (1970) Hybridization in Taraxacum. New Phytol 69:1103–1121. doi:10.1111/j.1469-8137.1970.tb02492.x
Richards AJ (1973) The origin of Taraxacum agamospecies. Bot J Linn Soc 66:189–211. doi:10.1111/j.1095-8339.1973.tb02169.x
Richards AJ (1997) Plant breeding systems, 2nd edn. Chapman and Hall, London
Sakata T, Higashitani A (2008) Male sterility accompanied with abnormal anther development in plants–genes and environmental stresses with special reference to high temperature injury. Int J Pl Developm Biol 2:42–51
Saxena KB, Sultana R, Mallikarjuna N, Saxena RK, Kumar RV, Sawargaonkar SL, Varshney RK (2010) Male-sterility systems in pigeonpea and their role in enhancing yield. Pl Breeding 129:125–134. doi:10.1111/j.1439-0523.2009.01752.x
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Pl Sci 3:175–180. doi:10.1016/S1360-1385(98)01235-7
Slade K, Rich TCG (2007) Pollen studies in British Hieracium sect. Alpina (Asteraceae). Watsonia 26:443–450
Sterk AA, Den Nijs JCM, Kreune K (1982) Sexual and agamospermous Taraxacum species in the Netherlands. Acta Bot Neerl 31:227–237
Tikhomirov V (2003) Generis Taraxacum Wigg. (Asteraceae) species nova e Belorussia. Novosti Sist Vyssh Rast 35:207–210
Urbanska K (1991) Pollen and male function in agamospermous plants. Pol Bot Stud 2:71–84
Urbanska K (1994) Pollen, mating and paternity in agamospermous angiosperms. Pl Spec Biol 9:57–67. doi:10.1111/j.1442-1984.1994.tb00083.x
Van Baarlen P, Van Dijk PJ, Hoekstra RF, De Jong JH (2000) Meiotic recombination in sexual diploid and apomictic triploid dandelions (Taraxacum officinale L.). Genome 43:827–835. doi:10.1139/g00-047
Van Baarlen P, De Jong JH, Van Dijk PJ (2002) Comparative cyto-embryological investigations of sexual and apomictic dandelions (Taraxacum) and their apomictic hybrids. Sex Pl Reprod 15:31–38. doi:10.1007/s00497-002-0132-x
Van der Hulst RGM, Meirmans P, Van Tienderen PH, Van Damme JMM (2004) Nuclear–cytoplasmic male-sterility in diploid dandelions. Heredity 93:43–50. doi:10.1038/sj.hdy.6800478
Van Dijk PJ (2003) Ecological and evolutionary opportunities of apomixis: insights from Taraxacum and Chondrilla. Philos T Roy Soc B 358:1113–1120. doi:10.1098/rstb.2003.1302
Van Dijk PJ (2009) Apomixis: Basics for non-botanists. In: Schön I, Martens K, van Dijk P (eds) Lost sex, Springer, New York, pp 47–62
Van Dijk PJ, Bakx-Schotmann TJM (2004) Formation of unreduced megaspores (diplospory) in apomictic dandelions (Taraxacum officinale, s.l.) is controlled by a sex-specific dominant locus. Genetics 166:483–492. doi:10.1534/genetics.166.1.483
Van Dijk PJ, Tas ICQ, Falque M, Bakx-Schotman T (1999) Crosses between sexual and apomictic dandelions (Taraxacum). II. The breakdown of apomixis. Heredity 83:715–721. doi:10.1038/sj.hdy.6886200
Van Dijk PJ, Van Baarlen P, De Jong JH (2003) The occurrence of phenotypically complementary apomixis-recombinants in crosses between sexual and apomictic dandelions (Taraxacum officinale). Sex Pl Reprod 16:71–76. doi:10.1007/s00497-003-0177-5
Van Soest JL (1965) Taraxacum sect. Palustria Dahlstedt. Acta Bot Neerl 14:1–53. doi:10.1111/j.1438-8677.1965.tb00178.x
Verduijn MH, Van Dijk PJ, Van Damme JMM (2004) The role of tetraploids in the sexual–asexual cycle in dandelions (Taraxacum). Heredity 93:390–398. doi:10.1038/sj.hdy.6800515
Vijverberg K, Van der Hulst RGM, Lindhout P, Van Dijk PJ (2004) A genetic linkage map of the diplosporous chromosomal region in Taraxacum officinale (common dandelion; Asteraceae). Theor Appl Genet 108:725–732. doi:10.1007/s00122-003-1474-y
Vijverberg K, Milanovic-Ivanovic S, Bakx-Schotman T, Van Dijk PJ (2010) Genetic fine-mapping of DIPLOSPOROUS in Taraxacum (dandelion; Asteraceae) indicates a duplicated DIP-gene. BMC Pl Biol 10:154. doi:10.1186/1471-2229-10-154
Whitton J, Sears CJ, Baack EJ, Otto SP (2008) The dynamic nature of apomixis in the angiosperms. Int J Pl Sci 169:169–182. doi:10.1086/523369
Acknowledgments
We thank Dr. Valery Tikhomirov (University of Minsk, Belarus) for confirming the taxonomical identification of Taraxacum belorussicum. This work was partially supported by the Polish Ministry of Science and Higher Education of Poland from the scientific statutory project of the Institute of Botany, Jagiellonian University; in the case of Agnieszka Janas, this study was funded in part by the Dean of the Faculty of Biology and Earth Sciences of Jagiellonian University (Project DS/MND/WBiNoZ/IB/II/2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Martin Lysak.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Janas, A., Musiał, K., Kościńska-Pająk, M. et al. Insights into developmental processes in anthers, ovaries, and ovules of Taraxacum belorussicum (Asteraceae-Cichorioideae) using DIC optics. Plant Syst Evol 302, 617–628 (2016). https://doi.org/10.1007/s00606-016-1288-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-016-1288-4