Abstract
An aptamer based assay is described for the determination of Salmonella typhimurium (S.typhimurium). A metal-organic framework-graphene composite of type UiO-67/GR is used as the substrate, and an aptamer-gold nanoparticles-horseradish peroxidase (Apt-AuNP-HRP) conjugate the signal amplification probe. A phosphate-terminal and partially complementary DNA (cDNA) of the aptamer is covalently bound to UiO-67/GR via the chemical complexation between phosphate and Zr-OH groups of UiO-67, and then S. typhimurium and cDNA will compete for the binding sites. The binding of Apt-AuNP-HRP to S.typhimurium leads to the formation of strong conjugates. The unbound signal probes then attach to the surface of a glassy carbon electrode via hybridization with cDNA. This generates a large current response (best measured at a potential as low as −0.02 V vs. saturated calomel electrode) under the catalytic action of HRP on the H2O2-hydroquinone system. Under the optimal conditions, the differential pulse voltammetric signal decreases linearly in the 2 × 101 – 2 × 108 cfu·mL−1 S.typhimurium concentration range, with a lower detection limit of 5 cfu·mL−1 (based on S/N = 3). The method was successfully applied to the detection of S. typhimurium in spiked milk samples.

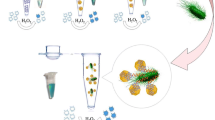
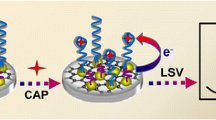
Schematic presentation of electrochemical determination of Salmonella typhimurium (S.typhimurium). A metal-organic framework (type UiO-67) and graphene (GR) composite were used as substrate, and gold nanoparticles carrying horseradish peroxidase (HRP) for signal amplification. HQ: hydroquinone; cDNA: complementary DNA of aptamer.





Similar content being viewed by others
References
Scharff RL (2015) State estimates for the annual cost of foodborne illness. J Food Prot 78(6):1064–1071. https://doi.org/10.4315/0362-028x.Jfp-14-505
Lee SC, Kim MS, Yoo KC, Ha NR, Moon JY, Lee SJ, Yoon MY (2017) Sensitive fluorescent imaging of Salmonella enteritidis and Salmonella typhimurium using a polyvalent directed peptide polymer. Microchim Acta 184(8):2611–2620. https://doi.org/10.1007/s00604-017-2240-1
Gonzalez-Escalona N, Brown EW, Zhang GD (2012) Development and evaluation of a multiplex real-time PCR (qPCR) assay targeting ttrRSBCA locus and invA gene for accurate detection of Salmonella spp. in fresh produce and eggs. Food Res Int 48(1):202–208. https://doi.org/10.1016/j.foodres.2012.03.009
Ahari H, Kakoolaki S, Anvar SAA (2017) Detection of Salmonella typhi using four developed kits of ELISA for cleaning in place purification. Int J Environ Sci Technol 14(10):2149–2154. https://doi.org/10.1007/s13762-017-1309-z
Vinayaka AC, Ngo TA, Kant K, Engelsmann P, Dave VP, Shahbazi M-A, Wolff A, Bang DD (2019) Rapid detection of Salmonella enterica in food samples by a novel approach with combination of sample concentration and direct PCR. Biosens Bioelectron 129:224–230. https://doi.org/10.1016/j.bios.2018.09.078
Lin D, Pillai RG, Lee WE, Jemere AB (2019) An impedimetric biosensor for E. coli O157:H7 based on the use ofself-assembled gold nanoparticles and protein G. Microchim Acta 186:169. https://doi.org/10.1007/s00604-019-3282-3
Majdinasab M, Hayat A, Marty JL (2018) Aptamer-based assays and aptasensors for detection of pathogenic bacteria in food samples. TrAC Trends Anal Chem 107:60–77. https://doi.org/10.1016/j.trac.2018.07.016
Felisilda BMB, Arrigan DWM (2018) Electroactivity of aptamer at soft microinterface arrays. Anal Chem 90(14):8470–8477. https://doi.org/10.1021/acs.analchem.8b01172
Negahdary M, Behjati-Ardakani M, Heli H (2019) An electrochemical troponin T aptasensor based on the use of a macroporous gold nanostructure. Microchim Acta 186:377. https://doi.org/10.1007/s00604-019-3472-z
Xu X, Ma X, Wang H, Wang Z (2018) Aptamer based SERS detection of Salmonella typhimurium using DNA-assembled gold nanodimers. Microchim Acta 185:325. https://doi.org/10.1007/s00604-018-2852-0
Wang XD, Dong J, Liu XY, Liu YZ, Ai SY (2014) A novel pH-controlled immunosensor using hollow mesoporous silica and apoferritin combined system for target virus assay. Biosens Bioelectron 54:85–90. https://doi.org/10.1016/j.bios.2013.10.051
Anik U, Timur S, Dursun Z (2019) Metal organic frameworks in electrochemical and optical sensing platforms: a review. Microchim Acta 186:196. https://doi.org/10.1007/s00604-019-3321-0
Liu W, Yin XB (2016) Metal-organic frameworks for electrochemical applications. TrAC Trends Anal Chem 75:86–96. https://doi.org/10.1016/j.trac.2015.07.011
Liu C, Yu LQ, Zhao YT, Lv YK (2018) Recent advances in metal-organic frameworks for adsorption of common aromatic pollutants. Microchim Acta 185:342. https://doi.org/10.1007/s00604-018-2879-2
Dhakshinamoorthy A, Asiri AM, Garcia H (2016) Metal-organic frameworks as catalysts for oxidation reactions. Chem Eur J 22(24):8012–8024. https://doi.org/10.1002/chem.201505141
Hu ZC, Deibert BJ, Li J (2014) Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem Soc Rev 43(16):5815–5840. https://doi.org/10.1039/c4cs00010b
Kumar P, Pournara A, Kim KH, Bansal V, Rapti S, Manos MJ (2017) Metal-organic frameworks: challenges and opportunities for ion-exchange/sorption applications. Prog Mater Sci 86:25–74. https://doi.org/10.1016/j.pmatsci.2017.01.002
Liu C, Dong J, Ning SX, Hou JY, Waterhouse GIN, Cheng ZQ, Ai SY (2018) An electrochemical immunosensor based on an etched zeolitic imidazolate framework for detection of avian leukosis virus subgroup J. Microchim Acta 185:423. https://doi.org/10.1007/s00604-018-2930-3
Dong X, Zhao GH, Liu L, Li X, Wei Q, Cao W (2018) Ultrasensitive competitive method-based electrochemiluminescence immunosensor for diethylstilbestrol detection based on Ru(bpy)(3)(2+) as luminophor encapsulated in metal organic frameworks UiO-67. Biosens Bioelectron 110:201–206. https://doi.org/10.1016/j.bios.2018.03.066
Bai Y, Dou YB, Xie LH, Rutledge W, Li JR, Zhou HC (2016) Zr-based metal-organic frameworks: design, synthesis, structure, and applications. Chem Soc Rev 45(8):2327–2367. https://doi.org/10.1039/c5cs00837a
Zhu X, Li B, Yang J, Li Y, Zhao W, Shi J, Gu J (2015) Effective adsorption and enhanced removal of organophosphorus pesticides from aqueous solution by Zr-based MOFs of UiO-67. ACS Appl Mater Interfaces 7(1):223–231. https://doi.org/10.1021/am5059074
Liu Y, Hou WJ, Xia L, Cui C, Wan S, Jiang Y, Yang Y, Wu Q, Qiu LP, Tan WH (2018) ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem Sci 9(38):7505–7509. https://doi.org/10.1039/c8sc02210k
Wang X, Wu M, Tang WR, Zhu Y, Wang LW, Wang QJ, He PG, Fang YZ (2013) Simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid using a palladium nanoparticle/graphene/chitosan modified electrode. J Electroanal Chem 695:10–16. https://doi.org/10.1016/j.jelechem.2013.02.021
Zuo YX, Xu JK, Zhu XF, Duan XM, Lu LM, Yu YF (2019) Graphene-derived nanomaterials as recognition elements for electrochemical determination of heavy metal ions: a review. Microchim Acta 186:171. https://doi.org/10.1007/s00604-019-3248-5
Musyoka NM, Ren JW, Langmi HW, North BC, Mathe M, Bessarabov D (2017) Synthesis of rGO/Zr-MOF composite for hydrogen storage application. J Alloys Compd 724:450–455. https://doi.org/10.1016/j.jallcom.2017.07.040
Yang Q, Wang J, Zhang W, Liu F, Yue X, Liu Y, Yang M, Li Z, Wang J (2017) Interface engineering of metal organic framework on graphene oxide with enhanced adsorption capacity for organophosphorus pesticide. Chem Eng J 313:19–26. https://doi.org/10.1016/j.cej.2016.12.041
Dong J, Zhao H, Xu MR, Ma Q, Ai SY (2013) A label-free electrochemical impedance immunosensor based on AuNPs/PAMAM-MWCNT-chi nanocomposite modified glassy carbon electrode for detection of Salmonella typhimurium in milk. Food Chem 141(3):1980–1986. https://doi.org/10.1016/j.foodchem.2013.04.098
Nguyen PD, Tran TB, Nguyen DTX, Min J (2014) Magnetic silica nanotube-assisted impedimetric immunosensor for the separation and label-free detection of Salmonella typhimurium. Sensors Actuators B Chem 197:314–320. https://doi.org/10.1016/j.snb.2014.02.089
Sheikhzadeh E, Chamsaz M, Turner APF, Jager EWH, Beni V (2016) Label-free impedimetric biosensor for Salmonella typhimurium detection based on poly pyrrole-co-3-carboxyl-pyrrole copolymer supported aptamer. Biosens Bioelectron 80:194–200. https://doi.org/10.1016/j.bios.2016.01.057
Jia F, Duan N, Wu SJ, Dai RT, Wang ZP, Li XM (2016) Impedimetric Salmonella aptasensor using a glassy carbon electrode modified with an electrodeposited composite consisting of reduced graphene oxide and carbon nanotubes. Microchim Acta 183(1):337–344. https://doi.org/10.1007/s00604-015-1649-7
Ranjbar S, Shahrokhian S, Nurmohammadi F (2018) Nanoporous gold as a suitable substrate for preparation of a new sensitive electrochemical aptasensor for detection of Salmonella typhimurium. Sensors Actuators B Chem 255:1536–1544. https://doi.org/10.1016/j.snb.2017.08.160
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21575042).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 833 kb)
Rights and permissions
About this article
Cite this article
Dai, G., Li, Z., Luo, F. et al. Electrochemical determination of Salmonella typhimurium by using aptamer-loaded gold nanoparticles and a composite prepared from a metal-organic framework (type UiO-67) and graphene. Microchim Acta 186, 620 (2019). https://doi.org/10.1007/s00604-019-3724-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3724-y