Abstract
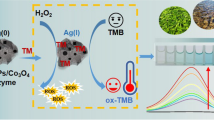
A new colorimetric aptasensor equipped with a novel composite of graphitic carbon nitride (g-C3N4) nanosheets and copper oxide(I) (Cu2O) nanocrystals is presented for Salmonella typhimurium (S .typhimurium). The dual-purpose structure of this composite simultaneously contributes to superb peroxidase-like activity and interaction with a label-free aptamer. Although g-C3N4@Cu2O effectively creates a visible blue color following the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in presence of hydrogen peroxide (H2O2), this catalytic activity of the composite severely decreases after the interaction with aptamers. In the presence of S. typhimurium in sample, aptamers bound to their specific target. Subsequently, g-C3N4@Cu2O catalytic activity was enhanced in proportion to S. typhimurium concentration. Under optimized conditions, this aptasensor exhibited an excellent detection performance in a range from 1.5 × 101 to 1.5 × 105 CFU/ml, with a detection limit of 15 CFU/ml. Besides, portable detection of S. typhimurium by paper-based model of this method operated successfully in just 6 min. Analysis of spiked milk samples revealed high potential of this method as a sensitive, rapid, and label-free promising tool for S. typhimurium detection.
Graphical abstract

A novel composite of g-C3N4 nanosheets and Cu2O nanocrystals was constructed through this study, which represented a collection of significant properties for designing an aptasensor. The simultaneous capability of this composite for peroxidase-like activity and interaction with aptamer led to design a fast accurate biosensor for detecting as low as 15 CFU/ml Salmonella typhimurium as one of the most important foodborne pathogens which is a persistent burden for societies.





Similar content being viewed by others

References
Holban AM, Grumezescu AM (2018) Microbial contamination and food degradation, eds. Academic Press, London, United Kingdom
Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM (2015) Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28:901–937. https://doi.org/10.1128/CMR.00002-15
Torpdahl M, Lauderdale T-L, Liang S-Y, Li I, Wei S-H, Chiou C-S (2013) Human isolates of Salmonella enterica serovar Typhimurium from Taiwan displayed significantly higher levels of antimicrobial resistance than those from Denmark. Int J Food Microbiol 161:69–75. https://doi.org/10.1016/j.ijfoodmicro.2012.11.022
Inbaraj BS, Chen BH (2016) Nanomaterial-based sensors for detection of foodborne bacterial pathogens and toxins as well as pork adulteration in meat products. J Food Drug Anal 24:15–28. https://doi.org/10.1016/j.jfda.2015.05.001
Guadarrama C, Villaseñor T, Calva E (2014) The subtleties and contrasts of the LeuO regulator in Salmonella Typhi: implications in the immune response. Front Immunol 5:581. https://doi.org/10.3389/fimmu.2014.00581
Pashazadeh P, Mokhtarzadeh A, Hasanzadeh M, Hejazi M, Hashemi M, de la Guardia M (2017) Nano-materials for use in sensing of salmonella infections: recent advances. Biosens Bioelectron 87:1050–1064. https://doi.org/10.1016/j.bios.2016.08.012
Srisa-Art M, Boehle KE, Geiss BJ, Henry CS (2018) Highly sensitive detection of Salmonella typhimurium using a colorimetric paper-based analytical device coupled with immunomagnetic separation. Anal Chem 90:1035–1043. https://doi.org/10.1021/acs.analchem.7b04628
Reta N, Saint CP, Michelmore A, Prieto-Simon B, Voelcker NH (2018) Nanostructured electrochemical biosensors for label-free detection of water-and food-borne pathogens. ACS Appl Mater Interfaces 10:6055–6072. https://doi.org/10.1021/acsami.7b13943
Ranjbar S, Shahrokhian S, Nurmohammadi F (2018) Nanoporous gold as a suitable substrate for preparation of a new sensitive electrochemical aptasensor for detection of Salmonella typhimurium. Sensors Actuators B Chem 255:1536–1544. https://doi.org/10.1016/j.snb.2017.08.160
Shahsavar K, Hosseini M, Shokri E, Ganjali MR, Ju H (2017) A sensitive colorimetric aptasensor with a triple-helix molecular switch based on peroxidase-like activity of a DNAzyme for ATP detection. Anal Methods 9:4726–4731. https://doi.org/10.1039/C7AY01381G
Kermani HA, Hosseini M, Miti A, Dadmehr M, Zuccheri G, Hosseinkhani S, Ganjali MR (2018) A colorimetric assay of DNA methyltransferase activity based on peroxidase mimicking of DNA template Ag/Pt bimetallic nanoclusters. Anal Bioanal Chem 410:4943–4952. https://doi.org/10.1007/s00216-018-1143-2
Dehghani Z, Hosseini M, Mohammadnejad J, Bakhshi B, Rezayan AH (2018) Colorimetric aptasensor for Campylobacter jejuni cells by exploiting the peroxidase like activity of Au@ Pd nanoparticles. Microchim Acta 185:448. https://doi.org/10.1007/s00604-018-2976-2
Dehghani Z, Hosseini M, Mohammadnejad J, Ganjali MR (2019) New colorimetric DNA sensor for detection of Campylobacter jejuni in Milk sample based on peroxidase-like activity of gold/platinium nanocluster. ChemistrySelect 4:11687–11692. https://doi.org/10.1002/slct.201901815
Abarghoei S, Fakhri N, Borghei YS, Hosseini M, Ganjali MR (2019) A colorimetric paper sensor for citrate as biomarker for early stage detection of prostate cancer based on peroxidase-like activity of cysteine-capped gold nanoclusters. Spectrochim Acta A Mol Biomol Spectrosc 210:251–259. https://doi.org/10.1016/j.saa.2018.11.026
Hosseini M, Aghazadeh M, Ganjali MR (2017) A facile one-pot synthesis of cobalt-doped magnetite/graphene nanocomposite as peroxidase mimetics in dopamine detection. New J Chem 41:12678–12684. https://doi.org/10.1039/C7NJ02082A
Naderi M, Hosseini M, Ganjali MR (2018) Naked-eye detection of potassium ions in a novel gold nanoparticle aggregation-based aptasensor. Spectrochim Acta A Mol Biomol Spectrosc 195:75–83. https://doi.org/10.1016/j.saa.2018.01.051
Xu H-H, Deng H-H, Lin X-Q, Wu Y-Y, Lin X-L, Peng H-P, Liu A-L, Xia X-H, Chen W (2017) Colorimetric glutathione assay based on the peroxidase-like activity of a nanocomposite consisting of platinum nanoparticles and graphene oxide. Microchim Acta 184:3945–3951. https://doi.org/10.1007/s00604-017-2429-3
Chinnappan R, AlAmer S, Eissa S, Rahamn AA, Salah KMA, Zourob M (2018) Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Microchim Acta 185:61. https://doi.org/10.1007/s00604-017-2601-9
Xu X, Ma X, Wang H, Wang Z (2018) Aptamer based SERS detection of Salmonella typhimurium using DNA-assembled gold nanodimers. Microchim Acta 185:7–325
Lee J, Jung J, Lee CS, Ha TH (2017) Design and optimization of an ultra-sensitive hairpin DNA aptasensor for Salmonella detection. RSC Adv 7:34933–34938. https://doi.org/10.1039/C7RA06000A
Mesgari F, Beigi SM, Salehnia F, Hosseini M, Ganjali MR (2019) Enhanced electrochemiluminescence of Ru (bpy) 32+ by Sm2O3 nanoparticles decorated graphitic carbon nitride nano-sheets for pyridoxine analysis. Inorg Chem Commun 106:240–247. https://doi.org/10.1016/j.inoche.2019.05.023
Salehnia F, Hosseini M, Ganjali MR (2017) A fluorometric aptamer based assay for cytochrome C using fluorescent graphitic carbon nitride nanosheets. Microchim Acta 184:2157–2163. https://doi.org/10.1007/s00604-017-2130-6
Nemati F, Zare-Dorabei R (2019) A ratiometric probe based on Ag2S quantum dots and graphitic carbon nitride nanosheets for the fluorescent detection of cerium. Talanta 200:249–255. https://doi.org/10.1016/j.talanta.2019.03.059
Fakhri N, Salehnia F, Beigi SM, Aghabalazadeh S, Hosseini M, Ganjali MR (2019) Enhanced peroxidase-like activity of platinum nanoparticles decorated on nickel-and nitrogen-doped graphene nanotubes: colorimetric detection of glucose. Microchim Acta 186:385. https://doi.org/10.1007/s00604-019-3489-3
Feng T, Chen X, Qiao X, Sun Z, Wang H, Qi Y, Hong C (2016) Graphene oxide supported rhombic dodecahedral Cu2O nanocrystals for the detection of carcinoembryonic antigen. Anal Biochem 494:101–107. https://doi.org/10.1016/j.ab.2015.11.004
Fakhri N, Hosseini M, Tavakoli O (2018) Aptamer-based colorimetric determination of Pb 2+ using a paper-based microfluidic platform. Anal Methods 10:4438–4444. https://doi.org/10.1039/C8AY01331D
Teengam P, Siangproh W, Tuantranont A, Vilaivan T, Chailapakul O, Henry CS (2017) Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal Chem 89:5428–5435. https://doi.org/10.1021/acs.analchem.7b00255
Odumeru JA, León-Velarde CG (2012) Salmonella detection methods for food and food ingredients. Salmonella-a dangerous foodborne pathogen. Rijeka, Croatia. InTec:373–392. https://doi.org/10.5772/29526
Joshi R, Janagama H, Dwivedi HP, Kumar TMAS, Jaykus L-A, Schefers J, Sreevatsan S (2009) Selection, characterization, and application of DNA aptamers for the capture and detection of Salmonella enterica serovars. Mol Cell Probes 23:20–28. https://doi.org/10.1016/j.mcp.2008.10.006
Hosseini M (2020) Fast and selective whole cell detection of Staphylococcus aureus bacteria in food samples by paper based colorimetric nanobiosensor using peroxidase-like catalytic activity of DNA-au/Pt bimetallic nanoclusters. Microchem J:105475. https://doi.org/10.1016/j.microc.2020.105475
Kuo M-Y, Hsiao C-F, Chiu Y-H, Lai T-H, Fang M-J, Wu J-Y, Chen J-W, Wu C-L, Wei K-H, Lin H-C (2019) Au@ Cu2O core@ shell nanocrystals as dual-functional catalysts for sustainable environmental applications. Appl Catal B Environ 242:499–506. https://doi.org/10.1016/j.apcatb.2018.09.075
Chen S, Yang X, Fu S, Qin X, Yang T, Man C, Jiang Y (2020) A novel AuNPs colorimetric sensor for sensitively detecting viable Salmonella typhimurium based on dual aptamers. Food Control:107281. https://doi.org/10.1016/j.foodcont.2020.107281
Oh SY, Heo NS, Shukla S, Cho H-J, Vilian ATE, Kim J, Lee SY, Han Y-K, Yoo SM, Huh YS (2017) Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-10188-2
Hou Y, Cai G, Zheng L, Lin J (2019) A microfluidic signal-off biosensor for rapid and sensitive detection of Salmonella using magnetic separation and enzymatic catalysis. Food Control 103:186–193. https://doi.org/10.1016/j.foodcont.2019.04.008
Wang L, Huo X, Zheng L, Cai G, Wang Y, Liu N, Wang M, Lin J (2020) An ultrasensitive biosensor for colorimetric detection of Salmonella in large-volume sample using magnetic grid separation and platinum loaded zeolitic imidazolate Framework-8 nanocatalysts. Biosens Bioelectron 150:111862. https://doi.org/10.1016/j.bios.2019.111862
Funding
The authors are grateful to the Research Council of University of Tehran for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 599 kb).
Rights and permissions
About this article
Cite this article
Tarokh, A., Pebdeni, A.B., Othman, H.O. et al. Sensitive colorimetric aptasensor based on g-C3N4@Cu2O composites for detection of Salmonella typhimurium in food and water. Microchim Acta 188, 87 (2021). https://doi.org/10.1007/s00604-021-04745-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04745-w