Abstract
Paper is a widely used support for use in devices for point-of-care testing (POCT) in clinical diagnosis, food safety monitoring and environmental pollution. Paper is inexpensive, biocompatible, biodegradable and allows a sample fluid to flow by capillary force. Numerous method have been developed recently for chemical modification of papers in order to introduce different functionalities. This review (with 148 refs.) summarizes the recent progress in paper-based POCT devices. The introduction summarizes the state of the art of paper-based POCT devices and the physicochemical properties of existing unmodified materials (including cellulose, cellulose-based composites, cotton fibers, glass fibers, nitrocellulose, thread). Methods for paper modification for sample pretreatment are summarized next, with subsections on sample storage and collection, sample separation, nucleic acid extraction and sample preconcentration. Another main section covers approaches for paper modification for improving POCTs, with subsections on assays for proteins, nucleic acids, drugs, ion and organic molecules. The advantages and disadvantages of these approaches are compared. Several tables are presented that summarize the various modification techniques. A concluding section summarizes the current status, addresses challenges and gives an outlook on future perspectives of POCTs.

This review summarizes the progress that has been made in paper based point-of-care testing (POCT) and lateral flow assays (LFAs), quite often by using advanced nanomaterials for paper modification.
Similar content being viewed by others
Introduction
Point-of-care testing (POCT), an affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free, and deliverable (ASSURED) technology [1], has utilized outside of clinical laboratory near the patient or bedside [2] and further found widespread applications in biomedical fields, including disease diagnosis, food safety monitoring and environmental pollution detection [3,4,5]. Paper, as a ubiquitous substance in daily life, has attracted increasing attention as a powerful microfluidic platform for POCT due to its low cost, biocompatibility, portability, biodegradability and capability to drive fluid flow by capillary force [6,7,8,9]. For detection of various targets using the paper-based POCT devices, development of different types of papers is needed.
Normally, samples contain multiple components and need pretreatment procedure to purify target before detection. Different types of papers have been directly used to fabricate paper-based devices for sample pretreatment and detection [10,11,12,13,14,15,16]. For instance, blood separation membranes with highly asymmetric pore structures was used to separate plasma from whole blood [17]. Fusion 5 membranes from Whatman® was used for nucleic acid extraction [18] based on the high affinity between the positively charged glass fibers of the Fusion 5 and negatively charged DNA. Nitrocellulose (NC) membranes, adsorption paper and glass fibers as the main materials were used to fabricate lateral flow assays (LFAs) test strips based on their physicochemical features (e.g., noncovalent interactions/electrostatic adsorption between the paper and chemical molecules) for detections of proteins [19], nucleic acids [15] and ions/chemical molecules [20]. However, these papers have limitations which restrict their further POCT applications [21]. For example, the limited range of sample volumes on one layer of blood separation membrane (10 μL [22]~100 μL [23]) cannot meet the requirements of the relatively low detection sensitivity of several assays [17]. The efficiency of Fusion 5 in extracting nucleic acid is low because of the nonspecific absorbance of cell debris and interferents (e.g., hemoglobin), which affects the extraction purity and thus the analytical result [21]. In addition, the detection sensitivity and specificity of LFAs are still poor because the biological molecules (e.g., DNA, antibodies and antigens) are immobilized on NC membranes based on the simple physical adsorption of chemical reagents, which causes the nonspecific adsorption issue [24, 25]. To improve the performance of the paper-based devices and overcome these challenges, modification approaches to prepare papers with more functions for POCT applications are needed.
With the recent advances in paper materials, micro/nanotechnologies and nanomaterials, various modification approaches have been developed and used to improve the performance of paper-based POCT devices (Fig. 1). For example, a chitosan-based modification technology was utilized to modify Fusion 5 paper to improve the adsorption performance of DNA on the paper [12]. Paper functionalized with zinc oxide nanorods was used to preconcentrate myoglobin with enhanced performance compared to that of the unmodified paper [13]. A chemoenzymatic modification technology was developed to prepare paper-based fluorogenic esterase biosensors [26]. A wax-based technology was applied to modify hydrophobic patterns and hydrophobic barriers on paper using a wax printer [27] and a pen-writing approach [28]. Different nanomaterials, such as quantum dots (QDs) [29,30,31,32,33], gold nanoparticles (AuNPs) [24, 34,35,36,37,38,39,40,41,42,43,44,45,46], AuNFsMBA@Ag [47] Cu-Pd/rGO nanoparticles [48], upconverting fluorescent nanocrystals [49], metal-organic framework [50], fluorescent nanoparticles [51], gold nanocage [52], gold nanorods [53], multi-walled carbon nanotubes and graphene oxide [54] and polymer nanoparticles [55], have also been used as reading signal in paper-based POCT devices. The functional papers prepared by these advanced modification approaches significantly extend the application potential of paper as the substrate of POCT devices.
Existing paper modification approaches for paper-based POCT. Different paper materials, including Fusion 5, filter paper, chromatography paper, cellulose paper, Whatman® No.1 filter paper and NC membrane, have been modified with various reagents for paper-based sample pretreatment and paper-based detection
Thus, considering the paper modification approaches are critical for improving the performance of paper-based POCT devices, it is urgently needed for a review to summarize and compare the existing modification approaches of paper. Previous reviews mainly focus on the working principles [56], fluid flow theory [57], inkjet-printed fabrication technology [58] and fabrication materials and methods [59] of paper-based POCT devices. So in this review, we first summarize the existing papers and their physicochemical properties. Then we introduce the paper modification approaches for sample pretreatment and sample detection, which are the two major steps of paper-based POCT, and then highlight modification processes for fabricating paper-based devices. The advantages and disadvantages of paper modification approaches are also compared. Finally, we discuss the challenges and future perspectives of paper modification technology for POCT.
Existing unmodified papers for paper-based POCT and their physicochemical properties
Sample analysis process normally includes two steps: sample pretreatment (e.g., sample collection and storage, sample separation, nucleic acid extraction and sample pre-concentration) and sample detection (e.g., nucleic acid detection, protein detection and ion/chemical molecule detection). Conventional methods for sample pretreatment and sample detection, such as centrifuge, thermocycling instrument, filter device, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), usually require large, expensive, nonportable and complex equipment. And professional and skilled workers are needed to purify samples and retain targets before downstream analysis. Hence, portable, cheap and easy-to-use devices are urgently needed, especially for the resource-limited situations. Since Whiteside’s group first used paper to fabricate paper-based microfluidic devices [60], various types of papers with biomolecule adsorption capability and porous structure have been invented and used to prepare low-cost, simple and portable paper-based microfluidic devices, especially for sample pretreatment and sample detection in the POCT field.
Physicochemical properties of existing unmodified papers
The physicochemical properties of paper are the key factors affecting the performance of paper-based devices [61, 62]. For example, the pore size, specific surface area and hydrophobicity/hydrophilicity of paper affect the capillary forces [63], adsorption ability and flow rate of the paper [64, 65]. Chemical groups on paper surface (e.g., hydroxyl and carboxyl groups) can help immobilize chemical reagents on paper to achieve chemical reactions [66]. Thus, to provide a reference and guidance for choosing proper materials for preparation of paper-based devices, various types of the papers (including cellulose, cotton fiber, glass fiber, nitrocellulose membrane, thread) and their physicochemical properties are first introduced. A material selection diagram according to the equilibrium time and adsorption capacity of the paper material is shown in Fig. 2.
Cellulose
Cellulose usually with diameter of 30-40 nm (up to ~100 nm) and specific surface area of 1.4 m2.g-1 is produced by plants or bacteria. Cellulose contains hydroxyl groups on its surface and has the properties of hydrophilicity, easy usability, high porosity, high mechanical strength and degradability [86, 87]. Cellulose can be made into cellophane films and has a porous structure to provide abundant binding sites for adsorption of biomolecules through functionalization [88]. Bacterial cellulose (BC), which is produced by bacteria and has natural biosorbent, renewable, biodegradable and biocompatible properties has emerged as a new type of cellulose paper for preparing paper-based POCT devices [89]. And the BC fiber has a size of 100 nm and high porosity of 92% [90,91,92]. The amino groups on the BC surfaces can be used to remove residues and adsorb biomolecules, including proteins (e.g., bovine serum albumen, BSA) and heavy metals (e.g., Pb2+, Cd2+ and Ni2+) [67]. The porous structure of BC can adsorb biomolecules based on chemical bonds or physical adsorption. BC can be used as a substrate in paper-based POCT devices for sample pretreatment and sample detection.
Cellulose-based composite materials
Cellulose-based composite papers are composed of hybrids of cellulose with various materials, such as Eichhornia crassipes/chitosan, graphene oxide, maleic and phthalic anhydride [68,69,70, 93, 94]. The physicochemical properties of these composite materials such as surface functional groups, specific surface area and hydrophilicity, are better than those of the pure cellulose. For example, E. crassipes/chitosan composite materials have hydroxyl groups and -CH groups on their surface and with specific surface areas ranging from 132.95 m2.g-1 to 172.49 m2.g-1 and adsorption abilities of up to 0.606 mg.g-1 at an equilibrium time of 180 min [93]. Graphene oxide/sawdust composites have both the advantages of graphene (e.g., abundant oxygen-containing groups) and cellulose (e.g., nontoxic, biodegradable, renewable and modifiable) and contain mainly C-C groups on their surface. Graphene oxide/sawdust composites have a specific surface area ranging from 124.92 m2.g-1 to 78.13 m2.g-1 and an adsorption capacity up to 158.98 mg.g-1 at 30 min [68]. Graphene oxide/microcrystalline cellulose aerogels, with the advantages of low density, high specific surface area and hydroxyl groups on their surface, show a high adsorption ability (up to 2630 mg.g-1) for organic pollutants (e.g., methylene blue) [69]. Graphene oxide/carboxymethyl cellulose monoliths with a unidirectional porous structure have a compressive strength of 70 kPa, surfaces with abundant hydroxyl and carboxyl groups, a highest porosity of 96% and an adsorption capacity ranging from 46.13 to 82.93 mg.g-1 [71]. Cellulose modified with maleic and phthalic anhydride displays better adsorption capacity than cellulose, with maximum adsorption capacities up to 370 mg.g-1 and 111 mg.g-1, respectively, by introducing carboxyl groups [70].
Cotton fiber
Cotton fiber, a natural material, has a specific surface area of 37 m2.g-1 and contains hydroxyl groups on its surface, which can be modified with nitric acid, amino or collagen to change its structure or surface chemical groups to improve the adsorption capacity for molecules (e.g., 2, 4-dichlorophenoxyacetic acid [72], active brilliant red X-3B dye [73] and proteins) on its surface [74, 75]. Additionally, the high hydrophilicity and high porosity of cotton fiber can provide a porous network architecture, which contributes to the high fluid flow driven by capillary force in cotton fiber.
Glass fiber
Glass fiber is a kind of synthetic fiber and is formed of silica-based thin strands. Glass fiber has a diameter ranging from 15.2 to 18.4 μm, a tensile strength ranging from 0.24 N to 0.4 N [95], a density ranging from 2.5 g.cm-3 to 2.7 g.cm-3 [95], and a high modulus of elasticity of 60 GPa [96]. Glass fiber has been widely utilized as a support matrix in paper-based devices for adsorption of biomolecules (e.g., proteins, nucleic acids and nanoparticles) [76]. This material has the advantages of good chemical resistance, good tensile strength, excellent dimensional stability and high insulation ability [97].
Nitrocellulose membranes
NC membranes are created by the reaction of cellulose and nitric acid [98]. The surfaces of NC membranes, containing the strong dipoles of nitrate groups, can interact with the dipoles of peptide bonds on a protein surface and then immobilize the protein on the NC membrane. Furthermore, NC membranes have a negative charge and can adsorb biomolecules with a positive charge (e.g., antibodies, antigens or other proteins) through electrostatic adsorption. HF-90, HF-135 and HF-180 are the three main types of NC membranes used in POCT. The porosities and pore sizes of HF-90, HF-135 and HF-180 are 80.65%, 79.54% and 78.67% and 10-55 μm, 10-30 μm and 9-14 μm, respectively. The adsorption capacities of NC membranes for proteins are affected by its pore size [77], and the maximum adsorption capacities of HF-180, HF-135 and HF-90 are 2038 mg.g-1, 1810 mg.g-1 and 1135 mg.g-1, respectively.
Thread
Threads (e.g., spun thread, cotton yarn and cotton thread) are composed of a tremendous number of fibers tightly bonded with one another to form a twisted structure [99]. Because of the high tensile strength and flexibility, easy operation and modification, good wicking capacity, absorption capacity and fluid capillary flow of thread, it with excellent potential to form hydrophilic or hydrophobic transport channels has been used as the support material of POCT devices [99, 100]. Moreover, the pores of flow channels in threads show a wide variety of interfiber gap sizes and woven/twisted interstitial porosities and spaces. Thus, the properties of threads are affected by many factors such as porosity and pore size. Additionally, the specific surface area of thread is limited, and these patterning and printing technologies are difficult to use for the fabrication of POCT devices [100].
Existing unmodified papers used in paper-based POCT
To satisfy the development of POCT technologies, different types of papers and nanomaterials have been used to fabricate paper-based devices, as illustrated in Table 1.
LFA strips, the most commonly used paper-based microfluidic devices for sample detection in POCT, mainly include adsorption pads, NC membranes, conjugate pads, sample pads and back pads [101, 105, 106]. In LFAs, NC membranes, based on a versatile porous material with nitrate groups on its surface, can immobilize nucleic acids, proteins and other biomolecules through hydrophobic, noncovalent or electrostatic interactions [98]. Adsorption pads can adsorb water based on their good hydrophilicity. Conjugate pads and sample pads can store colloidal gold particles and samples because of their good hydrophilic and release properties. The adsorption pad, NC membrane, conjugate pad and sample pad are pasted on the back pad. For example, for nucleic acid detection, NC membranes have been used as a reaction substrate of LFAs to immobilize nucleic acid sequences by streptavidin-biotin reactions because streptavidin can be directly adsorbed on NC membranes by physical adsorption [64, 102, 107] or UV-light cross-linking between two oligonucleotide sequences and the NC membrane [108]. For protein detection, NC membranes have been used to directly immobilize antibodies through physical adsorption [101]. Thus, the performance of LFAs is affected by several factors, including the properties of sample pad, conjugate pad, absorbent pad and signaling molecules.
In addition, filter paper has also been directly utilized to prepare paper-based channels to drive liquid flow through filter paper and reach the reaction area based on capillary force [21]. Additionally, Whatman® filter paper No. 1 has been used to fabricate paper-based devices to detect esterase [26]. Moreover, filter paper with chemical groups on its surface can immobilize chemical molecules through chemical reactions [109].
Besides the above mentioned paper materials, various types of nanomaterials, such as carbon dots (CD) [110, 111], silver nanoparticles (AgNP) [41], AuNPs [38, 39, 43, 112, 113] and AuNPs@SiO2 NPs [25] and other nanomaterials [48, 50, 55], have also been used as detection probes to generate colorimetric signals on the test strips of LFAs (Table 2). The nanoparticles on LFAs are modified with different biomolecules (e.g., antibody), which can immobilize on conjugation pad and interact with target and then bind the biomolecules on test line for detection.
Paper modification approaches for sample pretreatment
Biological samples generally contain complex components, which makes direct detection challenging. Thus, sample pretreatment procedure before detection, including sample storage and collection, sample separation, nucleic acid extraction and sample pre-concentration, is necessary. For the POCT applications, different types of papers modified with various reagents to change their physicochemical properties (e.g., chemical groups and surface charge) and to enhance their performances for sample pretreatment have been used to prepare the paper-based microfluidic devices, as summarized in Table 3. In the following section, we will introduce the main paper modification approaches for sample pretreatment.
Sample storage and collection
In most cases, biological samples (e.g., whole blood, tissue, saliva and urine) need be stored and transported at low temperature before analysis. Tubes, bottles and refrigerators are normally utilized to collect and store samples. Compared to these conventional methods, paper has the advantages of low cost, porous structure, portability and ease of use. Thus, the paper-based sample storage and collection methods have been developed and used for pretreatment of biological samples.
For storage of different biological samples (e.g., blood, tissues, cells and bacteria) on paper, various chemical reagents have been used to modify different kinds of papers to change the chemical groups on their surfaces. For example, commercial papers, such as FTA cards, Whatman® 903 protein saver cards, Whatman® 3MM filter paper and Nobuto filter paper, were modified with chemical reagents to protect nucleic acids or proteins from being destroyed by bacteria or ultraviolet light (Fig. 3a) [115,116,117, 120]. Chromatography paper modified with butylated hydroxytoluene was used to collect and store blood sample [118]. These modified papers allow storage and collection of various samples at room temperature and are compatible with several downstream extraction methods, e.g., RNeasy Mini Blood kit [115], making them suitable for POCT applications. The sample storage periods of these papers are different and depend on the chemical reagents used for modification. For instance, Whatman® 3MM filter paper and Whatman® 903 protein saver cards can store blood samples for up to 13 weeks at low temperatures (e.g., -80 °C, -20 °C and 4 °C) and at 35 °C [115]. Chromatography paper can store blood samples for up to 92 days at 4 °C or room temperature [117]. Nobuto filter paper can store samples for up to three months at room temperature [116]. FTA cards can store blood samples for up to 22 years and buccal samples for up to 12 years at room temperature. However, these commercial papers have not be fully used for the sample in-result out bedside diagnosis, because they still need combine with the downstream analysis technologies (e.g., PCR [12], RT-PCR [121]) to accomplish the testing step.
Sample separation
Variability of debris components (e.g., red blood cells, inorganic salts and metabolites) in samples (e.g., whole blood, urine and sweat) can affect the accuracy of analytical results. Thus, sample separation is a necessary step to obtain targets from complex samples. But the most used lab method for sample separation, i.e., centrifugation, is expensive and not widely accessible for out-of-lab circumstance.
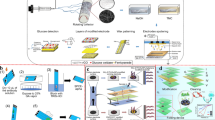
To realize the inexpensive and fast sample separation process, paper modification approaches have been developed for sample separation. For instance, in a thread-based device composed of cotton thread, holder and glass, ethylenediaminetetraacetic acid (EDTA) was modified on cotton thread to wick whole blood samples and separate plasma (Fig. 3b) [119]. The working principle of this device is that the scouring pre-treatment using Na2CO3 solution is used to remove the natural wax of cotton thread and increase the wicking properties of material and the absorbability of the reagent into the thread. After this process, the surfaces of the cotton thread and polymer have positive charges and hydroxyl groups, respectively, which can induce blood clotting on their surfaces. After modification of EDTA, the cotton thread can inhibit blood clotting process by activating platelet activity. The EDTA-treated cotton thread device has lower detection limit (114 mg.L-1) than that of the conventional blood analysis (133 mg.L-1). It is a low-cost, accurate and fast analytical platform for POCT in resource-limited settings. However, the performance of this device is influenced by temperature and storage time. In addition, chromatography paper modified with wax was used to prepare paper-based blood plasma separation device (Fig. 4a) [17]. This paper-based device is consisted of wax and chromatography paper, including the plasma separation zone in the center and the test area in the periphery. In the modification process, wax patterns designed by illustration software (e.g., CAD software) were printed on paper using a solid-ink printer. Then, the wax-printed paper was placed on a hotplate to melt the wax and create hydrophobic barriers which spans the device [17]. After patterning, the paper was modified with agglutinating antibodies and colorimetric chemical reagents by physical adsorption. The as-prepared device is capable of separating blood plasma based on the agglutination technique of red blood cell in the plasma separation area integrated into the device and detecting glucose for medical applications at home. However, for the application of this device, the expensive wax printer is still needed and the antibodies can easily denature, which affects the accuracy of detection result.
In another example, a paper origami-based electrophoretic device (oPAD-Eps) was fabricated by patterning wax on a slip layer and origami paper (Whatman® grade 1 paper) using CorelDraw software and a wax printer (Fig. 4b) [11]. Since the molecules can move through the paper-based substrate of oPAD-Eps device based on electrophoresis under an external electric field, the oPAD-Eps can separate fluorescent molecules, e.g., Ru(bpy)3Cl6, 4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a, 4a-diaza-s-indacene, 8-methoxypyrene-1,3,6-trisulfonic acid trisodium salt, 1,3,6,8-pyrenetetrasulfonic acid tetrasodium salt, rhodamine 6G, methylene blue and rhodamine B [11]. Thus, this sample pretreatment device can be further integrated with other paper-based detection devices for fluorescent detection. However, it is difficult to be used for bedside diagnosis because its needs of complex fabrication process and extra electric field. Additionally, the modification technology has a high cost because of the use of an inkjet printer.
Nucleic acid extraction
For detection of nucleic acids, nucleic acid extraction is a key step in isolating targets (e.g., DNA, RNA) from complex samples before analysis. However, the conventional extraction methods always include lysis, washing and elution procedures, which are complex, time consuming and not suitable for POCT applications.
To achieve rapid and simple extraction of nucleic acid, several papers (e.g., Fusion 5, glass fiber) have been used. However, the extraction efficiencies of these papers are still poor due to the nonspecific absorbance of cell debris and interferents (e.g., hemoglobin) on paper, which affects the extraction purity and sensitivity of downstream analysis. To enhance the extraction efficiency, several modification approaches have been utilized to change the surface charges of paper for nucleic acid extraction. For example, chitosan was used to modify Fusion 5 for paper-based DNA extraction (Fig. 5a) [12]. The device is composed of Fusion 5, PDMS membrane and PMMA. Fusion 5 was inserted into PDMS membrane and then sandwiched by two PMMA layers. In the modification process, Fusion 5 was modified sequentially by oxygen plasma activation, chitosan treatment, deionized water washing and vacuum drying. The capture principle of DNA in the modification process is based on the physical entanglement of long-chain DNA molecules and the fiber matrix and electrostatic adsorption between DNA and the chitosan-modified filter fibers. With this modification, the capture efficiencies of K562 human genomic DNA (up to 98%) and bacteriophage λ-DNA (up to 95%) are significantly improved. Fusion 5 can also preconcentrate λ-DNA from a diluted sample by more than 30-fold. But on the other hand, the extraction efficiency of nucleic acids of this device is affected by pH. The device requires syringe pump and valve to achieve automated extraction of DNA from a small volume of sample, indicating that it still needs improve its portability for bedside diagnosis. In addition, Whatman® No. 1 filter paper was modified with spermine, polyvinylpyrrolidone (PVP) 40 and cationic polymers (e.g., polyethylenimine (PEI), dopamine, 3-aminopropyl trimethoxysilane (APTMS) and chitosan) to bind DNA/RNA from animal, plant and microbe samples (Fig. 5b) [109]. In the binding process, the surface of the modified filter paper has a positive charge and can bind negatively charged DNA by electrostatic adsorption. The binding ability of nucleic acids on the chitosan- and PEI-modified filter paper is better than that of other compounds. The cellulose dipstick is composed of a wax impregnated handle and nucleic acid binding region. The cellulose-based dipstick can extract nucleic acid within 30 second without any pipette or electrical equipment and can combine with simple portable amplification device for POCT applications. In short, these modification approaches for filter paper can improve the extraction efficiency of DNA and can be integrated into paper-based devices for the rapid testing of nucleic acids in POCT.
Sample preconcentration
At the early stage of diseases, biomarkers with low concentrations are present in samples. The rapid detection of targets with low concentrations still meets challenges. Sample preconcentration process before detection is thus needed. Conventional preconcentration methods (e.g., centrifugation and filtration) are complex, expensive and time-consuming, making them difficult to be integrated into the POCT devices.
To address this issue, various fast, portable and inexpensive technologies for sample preconcentration have been developed. For instance, Whatman® No. 1 filter paper modified with zinc oxide nanorods using a hydrothermal method was developed to fabricate paper-based ELISA device for preconcentration of myoglobin (Fig. 6) [13]. The ZnO nanostructured-paper composite can form the oriented micro- or nanostructures on paper to provide the high surface area of binding sites, which contribute to the immobilization of biomolecules. And the modified paper treated with 3-APTES through salinization can increase the antibody binding sites (amides) on the paper surface to capture the target protein (myoglobin) for protein preconcentration. This device can detect myoglobin based on the antigen-antibody interaction. And the ELISA testing result of this device indicates that the preconcentration efficiency of biomarkers from a diluted solution (myoglobin < 50 nM) was enhanced 3-fold compared to that of the unmodified paper. However, the ZnO nanostructure-paper composite cannot independently use for POCT because it needs to combined with other biosensors.
Paper modification approaches for preconcentration. Paper modified with zinc oxide nanorods for Myoglobin pre-concentration [13]
The above examples show that various papers have been modified with different chemical reagents for sample pretreatment. These modification approaches have potential for fabricating functional devices with hydrophobic patterns and enhancing the performance of paper-based devices.
Paper modification approaches for sample detection
For the rapid detection of different targets (e.g., proteins, nucleic acids and ions/chemical molecules), various paper modification approaches have been used to improve the detection efficiency by changing the physicochemical properties of papers (e.g., chemical groups, hydrophobicity/hydrophobicity, wet strength and surface charge) (Table 4).
Protein detection
Proteins in whole blood, urine, saliva and sweat have been widely used as biomarkers for detection of various diseases [137]. Paper-based devices have been developed and utilized to detect different proteins [27, 35, 138]. However, the low detection efficiency of the paper-based devices limits their further applications in POCT.
To address this, different modification approaches have been developed to change the features of papers, including the surface functional groups, wet strength and specific surface area. For example, Whatman® chromatography paper was modified with carboxymethyl cellulose (CMC) to form a linker for immobilizing biomolecules in a hydrophilic barrier made of wax as reaction region to promote the functionality and stability of paper-based ELISA devices (Fig. 7a) [122]. The device was used to detect tuberculin based on the antigen-antibody interaction, and its hydrophilicity increased by CMC can reduce non-specific binding of random proteins to improve its detection sensitivity. In the modification process, 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) was crosslinked with the CMC-modified cellulose surface to produce NHS ester groups, which can enhance the specific binding between the paper substrate and the ligands or proteins by covalent conjugation. This modification technology can achieve a detection limit of 0.03 ng.mL-1 for tuberculin protein. The CMC-modified paper shows potential as an equipment-free diagnostic platform. However, the higher density of antibody attachment on paper substrate can increase the background noise and wax printer is expensive. Additionally, Whatman® chromatography paper was modified with polyamidoamine starburst dendrimer (PAMAM dendrimer) to fabricate a paper-based device for the detection of telomerase activity (Fig. 7b) [123]. The modification approach functionalized the paper surface with amino groups, which can improve the efficiency of biomolecules immobilization. The paper-based device was fabricated by hand drawing with using a template and was used to detect telomerase activity based on the hybridization of Cy5 modified single strand DNA probes with telomerase extension products. The PAMAM-based device is a simple and amplification-free fluorescence assay, making it suitable for disease diagnosis at bedside. However, this modification is expensive. Additionally, a novel peptide of diphenylalanine (FF) was used to modify Whatman® chromatography paper to prepare nanocomposite electrodes for detection of alpha-fetoprotein (AFP) (Fig. 7c) [124]. The device is composed of a lower sheet of plastic and an upper layer of cellulose paper modified with silver-graphene printed electrodes and uses antibody captured target protein to generate electrical response relevant to the concentrations of target protein. In the modification process, FF not only enhances the stability of immobilized antibodies via amine-aldehyde reactions, but also increases the wet strength of paper by forming paper-plastic integrated chips. The paper-based device can screen AFP with a range from 1 ng.mL-1 to 104 ng.mL-1 and achieve a detection limit of 10 ng.mL-1. It can be integrated into a miniaturized portable device as a paper-based biosensor for POCT. However, it still requires small amounts of reagents (e.g., hexafluoroisopropanol) compared to other methods.
Paper modification approaches for protein detection. a. Cellulose paper modified with CMC through EDC/NHS crosslinked for tuberculin-purified protein detection [122]. b. Paper modified with PAMAM dendrimer for telomerase activity detection [123]. c. Cellulose modified with FF for Alpha-fetoprotein detection [124]
Similarly, chromatography paper was modified with chitosan and glutaraldehyde to enhance the immobilization efficiency of antibody (e.g., ubiquitin or enhanced green fluorescent protein, cherry protein) on paper in the paper-based ELISA device (Fig. 8a) [125]. The device was fabricated by attaching paper disk to a plastic strip using double-sided tape and then building the straw region on the paper surface. And the modification process changes the hydrophobicity of the paper surface with varying the amounts of chitosan-glutaraldehyde. Based on the antigen-antibody interaction on the paper substrate, the target antibodies can be captured by protein, which leads to a color change on the paper. The testing results showed that the linear range of the targeted antibodies was 0.5 nM to 15 nM. Such modification technology is easy to perform without need of any special reagent or instrumentation and can also be used for paper-based enzyme and DNA assays based on the covalent coupling of enzymes and DNA molecules, making the paper-based device suitable for more POCT applications. However, the reactivity of glutaraldehyde and the zeta potential of chitosan are influenced by pH, which can affect the immobilization efficiency of protein on the paper surface. In another example, filter paper was modified with the amino-functionalized mesoporous material Santa Barbara Amorphous (SBA-15) to achieve the fluorescent detection of gliadin (Fig. 8b) [128]. The paper-based device used wax to prepare hydrophobic barriers to form microzone for reaction and mesoporous material to modify paper to provide more aldehyde groups on paper to enhance its immobilization efficiency of biomolecules. The modification process can improve the immobilization capacity of anti-gliadin antibodies on the paper surface by increasing the specific surface area of nanoporous structures and achieving the amino functionalization of SBA-15. The device achieves a detection limit of 0.025 mg.kg-1, good reproducibility and detection stability. Whereas, the synthesis and functionalization processes of SBA-15 are time consuming and complex. And the paper-based device is not suitable for fast detection of gliadin at resource-limited settings because it need to use extra equipment for analysis of fluorescent signal.
Paper modification approaches for protein detection. a. Paper modified with chitosan for protein detection [125]. b. Paper modified with SBA-15 technology for gliadin detection [128]. c. Thread modified with polysiloxanes technology for Salmonella enterica Serotype Enteritidis detection [129]. d. Dipropylene glycol methyl ether acetate containing 20% acrylic polymer is printed on the surface of the NC membrane to form barrier to achieve multi-step operations [130]
Moreover, paper and cotton thread were modified with hydrophobic reagents to enhance the detection efficiency of protein by delaying sample fluid flow rate. For example, polysiloxanes was modified on the surface of cotton thread and the fabricated cotton thread-based device was used to detect Salmonella enterica Serotype Enteritidis based on the antigen-antibody reaction (Fig. 8c) [129]. Similar with the fabrication of LFA, the thread-based device is composed of thread, adsorption pad, conjugation pad, sample pad and back pad. It uses antigen–antibody reaction to finish detection and incorporates polysiloxanes to change the hydrophobic of paper-based channel. The modification process used polysiloxanes to reduce the pore size and porosity of the cotton thread by forming hydrophobic barriers on the cotton thread. The detection limit is enhanced approximately 10-fold compared to that of the unmodified thread-based device. However, the preparation of this modified reagent is complex. Additionally, dipropylene glycol methyl ether acetate containing 20% acrylic polymer was modified on a NC membrane surface to create hydrophobic patterns, which can decrease the sample fluid flow and automate the multistep analysis on the paper-based ELISA device (Fig. 8d) [130]. The device was fabricated by printing dipropylene glycol methyl ether acetate containing 20% acrylic polymer on NC membrane to prepare hydrophobic barrier to form different regions, including non-delayed channel, a wax-delayed channel a test zone and a control zone. As an automated, one-step and portable paper-based ELISA device, it can be used for bedside diagnosis. However, this modification process needs extra equipment (e.g., wax printer). In a word, the performances of these modification approaches can be further enhanced by optimization of the modification processes with simpler procedures and equipment.
Nucleic acid detection
Nucleic acids, as one of the most fundamental biological substances in all organisms, have been used as typical biomarkers [34]. Detection of nucleic acids plays an important role in disease diagnosis. Unmodified paper cannot achieve high performance in nucleic acid detection due to its limited physicochemical properties. For example, unmodified Fusion 5 has low extraction efficiency of nucleic acid due to the higher adsorption ability of nonspecific biomolecules on its surface [18, 21]. The modification approaches of papers are thus needed for developing the paper-based nucleic acid detection devices.
For example, Whatman® No. 1 filter paper was modified with 1,4-phenylenediisothiocyanate and the fabricated paper device was used to detect nucleic acid based on the DNA hybridization reaction (Fig. 9a) [108]. In the modification process, the surface of Whatman® No. 1 filter paper was activated with aminated xyloglucan and xyloglucan to change the chemical groups on the fibers to react with aminated DNA without changing the morphology of fibers. The functionalized filter paper can accurately differentiate sequence-tagged amplicons of canine and human DNA in mock forensic samples. Moreover, such paper as a rapid and specific diagnostics can be used in real-life situations. In addition, papers modified with hydrophobic reagents were used to improve their detection efficiencies for nucleic acid detection. For instance, cellulose paper was modified with wax to form hydrophobic and insulating patterns on paper sheet and the unprinted wax area on paper with good hydrophilicity and porous structure was used as reaction area for the target in samples. Then the Ag/AgCl ink was screen-printed on the paper surface as electrodes to form paper-based electrochemical DNA assay (Fig. 9b) [131]. The prepared paper-based DNA assay was successfully used to electrochemically detect the concentrations of DNA in samples. It shows not only good detection sensitivity but also the capability for integration into emerging electronic devices for the development of high-throughput POCT devices in resource-limited settings. However, this process still needs extra wax printer. Besides wax, polydimethylsiloxane (PDMS) was also added on the NC membrane surface of LFA test strip to create hydrophobic barrier to delay fluid flow (Fig. 9c) [14]. The detection sensitivity of the prepared LFA for nucleic acids based on the nucleic acid hybridization reaction was improved 2-fold over that of the unmodified LFAs. Furthermore, the combination of a shunt and PDMS barrier in the LFAs yielded a 10-fold improvement in detection sensitivity over that of the unmodified LFAs. It can be utilized for bedside diagnosis or resource-limited settings.
Paper modification approaches for nucleic acid detection. a. 1,4-phenylene-disothiocyanate-based modification technology for nucleic acid extraction [108]. b. Wax-based modification technology is used to form hydrophobic and insulating patterned paper sheet for screen-printing electrodes in paper-based electrochemical DNA assay [131]. c. PDMS-based modification technology is used to fabricate the hydrophobic barrier on the NC membrane for improving the sensitivity of strip [14]
Drug detection
Drugs have been extensively utilized to treat, control and prevent diseases in human or animals. At present, various paper-based devices have been developed to detect drug concentrations in samples. But the low detection sensitivity of the paper-based devices is still a problem during their applications.
To handle this problem, several modification approaches by changing the surface groups on papers have been developed and utilized to improve the performance of the paper-based devices. For example, cellulose paper was modified with 3-triethoxysilylpropylamine (APTES) and the prepared paper-based test strip was used to detect oxitetracycline (OXY) through observing color change (Fig. 10a) [132]. The modification method can aminate the paper surface using APTES based on the self-assembly approach, because the amine group can bind metal ions (Fe(III) and Cu(II)) to form color. The detection concentration of OXY using this device is as low as 30 ng.mL-1. This device is inexpensive, equipment-free and environmental-friendly, making it suitable for resource-limited setting. However, this modification process needs complex approach and is influenced by the reaction time of APTES. Furthermore, Whatman® chromatography paper was modified with multiwalled carbon nanotubes (MWCNTs)/thionine (THI)/gold nanoparticles (AuNPs) nanocomposites, which function as the screen-printed working electrodes on paper for the detection of 17β-estradiol (Fig. 10b) [133]. The prepared paper-based device was used to detect 17β-estradiol based on electronic signal response. The modification process increases the specific surface area, which can improve the immobilization capacity of 17β-estradiol antibodies, allowing a higher density load of electroactive materials on paper surface to enhance the electrochemical current. The fabricated paper device has a wide linear detection range of 10 pg.mL-1 to 100 ng.mL-1 and a detection limit of 10 pg.mL-1. To achieve the really POCT, it still needs to develop a wireless portable electrochemical detector to combine with this device. However, the synthesis and functionalization of MWCNTs/THI/AuNPs is time consuming and complex.
Paper modification approaches for drug detection. a. Cellulose paper modified with APTES to prepare paper-based color test strip for oxitetracycline (OXY) detection [132]. b. Whatman® chromatography paper modified with MWCNTs/THI/ AuNPs Nano composites to prepare the screen-printed working electrodes for 17β-estradiol detection [133]
Ion/other chemical molecule detection
Heavy metals and toxic chemical molecules in drinking water threaten human health worldwide. Various novel paper-based devices have been fabricated and used for ion/chemical molecule detection. However, the low detection sensitivity of these devices is still a challenge for their further applications.
To handle this problem, various modification approaches have been utilized to change the surface chemical groups and hydrophilic/hydrophobic features of paper for ion/chemical molecule detection. For instance, APTMS, 3-triethoxysilylpropyl succinic anhydride (TESPSA) and mercaptopropyl trimethoxysilane (MPTMS) were modified on Whatman® chromatography paper by depositing vaporization for achieving multiplex detection of heavy metals (Fig. 11) [134]. The modification process immobilized amine groups, carboxyl groups and thiol groups on the surface of chromatography paper by condensation reactions. Three chromogenic reagents that reacted strongly with Ni(II), Cr(VI) and Hg(II) were covalently coupled to these functional groups, leading to color change of paper based on metal complexation reaction. The detection limits of the prepared devices are 0.24 ppm for Ni(II), 0.18 ppm for Cr(VI) and 0.19 ppm for Hg(II), respectively. It provides a simple and reliable analytical tool for POCT in low-resource settings. However, the uniformity of the modification approach need be further improved. In addition, filter paper modified with chitosan was also used to fabricate a colorimetric test strip for detection of mercury (Hg(II)) (Fig. 12) [29]. The paper test strip was fabricated by incorporating silver-doped CdS dots capped with mercaptoacetic into chitosan-coated filter paper, which can make a visualized color change from yellow to deep brown when Hg(II) ions were captured by the mercaptoacetic acid on CdSAg. The modification process enhanced the stability of CdSAg QDs immobilization and accelerated the process of colored adduct formation. The detection limit of the paper test strip for Hg(II) was 124 μM. It is a rapid and portable diagnostic, making it in real-time detection. However, this modification process is influenced by the chitosan concentration. Moreover, BSA was utilized to tune the wettability of Whatman® chromatography paper and the BSA-modified chromatography paper was used as a substrate of patterned paper assay by simple writing and stamping patterns on its surface (Fig. 13) [135]. This modification technology achieved the position control and spatial confinement of assay regions via the low solubility of the colored product. And the fabricated paper assay was successfully used to perform multi-target detections of Cu2+ and Ni2+ based on the precipitation reaction. It provides a portable diagnosis tool for POCT. However, this modification process is affected by the reaction temperature.
Paper modification approaches for ion/other chemical molecule detection. Whatman® chromatography paper modified with APTMS, TESPSA and MPTMS for Ni(II), Cr(II) and Hg(II), respectively [134]
Paper modification approaches for ion/other chemical molecule detection. Filter paper modified with chitosan has been used to fabricate a colorimetric test strip for the detection of mercury (Hg(II)) [29]
Paper modification approaches for ion/other chemical molecule detection. Whatman® chromatography paper modified with BSA for multi-targets detection of Cu2+ and Ni2+ [135]
In addition, the wax patterned filter paper modified with cobalt(II) phthalocyanine/ionic liquid/graphene composite (CoPc/IL/G) was used to fabricate an electrochemical paper-based assay for glucose detection (Fig. 14) [136]. The paper-based assay was fabricated by printing wax, CoPc/IL/G composite solution onto filter paper to prepare reaction regions and electrodes and depended on electronic signal to achieve detection. The modification process conferred the paper-based electrode excellent conductivity and fast electron transfer kinetics compared to those of the unmodified paper-based electrodes by enhancing the specific surface area of the paper-based material. The detection limit of glucose of this assay is 0.67 μM, and the linear dynamic ranges are 0.01 μM - 1.3 μM and 1.3 μM - 5.0 μM for low and high concentrations of glucose. To achieve the reality of POCT, the portable electrochemical detection device will be further developed. However, this modification technology requires multistep modification.
Paper modification approaches for ion/other chemical molecule detection. Filter paper after wax patterned modified with CoPc/IL/G for the glucose detection [136]
Based on the above summary, various chemical reagents, including CMC, PAMAM dendrimers, FF, chitosan, SBA-15, polysiloxanes, acrylic polymers, 1,4-phenylenediisothiocyanate, wax, PDMS, APTMS/TESPSA/MPTMS, APTES, CoPc/IL/G, MWCNTs/THI/AuNPs and BSA, have all been used to modify different types of papers to improve their detection efficiencies of nucleic acids, proteins, drugs and ions/other chemical molecules, respectively. Among these reagents, CMC, PAMAM dendrimers, 1, 4-phenylenediisothiocyanate and APTMS/TESPSA/MPTMS can change the chemical groups on the paper surface. Polysiloxanes can change the hydrophilic/hydrophobic nature of thread. Chitosan, FF and BSA can change the wet strength of paper. Wax, PDMS and acrylic polymers can penetrate into paper to form hydrophobic barriers. CoPc/IL/G and MWCNTs/THI/AuNPs can improve the specific surface area of paper. SBA-15 was used to modify paper by changing the specific surface area and altering the surface chemical groups on the paper. However, to alter other features of paper (e.g., porosity and pore size), the types of chemical reagents for modification are still limited.
Conclusion and future perspectives
Papers with different physicochemical properties have been utilized to fabricate various paper-based devices and used for sample pretreatment and sample detection in the POCT field. Considering that the performance of the paper-based devices with using the unmodified papers is still not satisfactory, many modification approaches have been utilized to tune the physicochemical features of paper materials, such as chemical groups, surface charge, hydrophilicity/hydrophobicity, wet strength and hydrophobic patterns/barriers. However, even the most common modification approaches are also associated with some limitations and cannot be widely used for batch preparation of paper-based devices. For example, experimental results often lack theoretical verification, such as the theory of fluid flow and the mass transfer/bioreactions inside the paper. Thus the mathematical models should be developed to analyze the convection-diffusion-reaction process in paper before and after modification [14, 21, 139, 140]. And the model results should be compared with the experimental results. In addition, to further understand the regulatory mechanism of modification reagents on paper, the physicochemical properties of paper before and after modification need be measured [141]. Furthermore, most chemical modification approaches are carried out on the commercial papers, resulting in the poor uniformity of structure due to artificial operation [29]. To improve the poor uniformity of structure, new paper modification approaches based on the papermaking process, such as addition of chemical reagents during the pulping process [142], can be used to produce special functional papers. Finally, hydrophobic patterning and barrier modification approaches will be further developed for integration with paper-based electronic chips [143].
Further effort will focus on developing the novel cellulose-based composite materials with programmable and tunable physicochemical properties for the fabrication of paper-based devices. To realize the practical use of paper-based devices and LFAs in POCT, the existing mature modification approaches (e.g., wax-based modification approach [144, 145] and chitosan-based modification approach [12, 109]) will step into the market. Furthermore, to fabricate paper-based integrated electronic chips, single paper material with multifunctional areas will be developed using different modification approaches. To integrate the extra criterion of R (real-time connectivity) and E (ease of specimen collection and environmental friendliness) into the ASSURED of POCT device and then create a new standard of REASSURED additionally, POCT devices should incorporate environmental-friendly modification reagents (e.g., chitosan [146]) to change the physiochemical feature of paper materials. Finally, paper-based modification approaches will play a greater role in POCT. For example, novel properties paper will be used for POCT detection (e.g., polydiacetylene-based paper assay shows chromatic properties and then it can be used for naked-eye detection of pandemic influenza a virus [147]). Composites materials can be utilized to increase the features of papers (e.g., graphene oxide/thionine/gold nanoparticles nanocomposites have been used for increase the adsorption ability of biomolecules on paper for cancer antigen 125 detection [148]).
References
Land KJ, Boeras DI, Chen XS, Ramsay AR, Peeling RW (2019) REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat Microbiol 4(1):46–54. https://doi.org/10.1038/s41564-018-0295-3
Hudson K (2005) Get bedside results with point of care testing. Nurs Manag 36:45–46. https://doi.org/10.1097/00006247-200501000-00012
Gootenberg JS, Abudayyeh OO, Zhang F (2018) Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 360(6387):439–444. https://doi.org/10.1126/science.aaq0179
Tapper EB, Parikh ND, Waljee AK, Volk M, Carlozzi NE, Lok AS (2018) Diagnosis of minimal hepatic encephalopathy: a systematic review of point-of-care diagnostic tests. Am J Gastroenterol 113(4):1–10. https://doi.org/10.1038/ajg.2018.6
Reddy B, Hassan U, Seymour C, Angus DC, Isbell TS, White K, Weir W, Yeh L, Vincent A, Bashir R (2018) Point-of-care sensors for the management of sepsis. Nat Biomedical Eng 2(9):640–648. https://doi.org/10.1038/s41551-018-0288-9
Yang MZ, Zhang W, Yang JC, Hu BF, Cao FJ, Zheng WS, Chen YP, Jiang XY (2017) Skiving stacked sheets of paper into test paper for rapid and multiplexed assay. Sci Adv 3(12):eaao4862. https://doi.org/10.1126/sciadv.aao4862
Lin BQ, Guan ZC, Song YL, Song E, Lu ZF, Liu D, An Y, Zhu Z, Zhou LJ, Yang CY (2018) Lateral flow assay with pressure meter readout for rapid point-of-care detection of disease-associated protein. Lab Chip 18(6):965–970. https://doi.org/10.1039/c8lc00010g
Brangel P, Sobarzo A, Parolo C, Miller BS, Howes PD, Gelkop S, Lutwama JJ, Dye JM, McKendry RA, Lobel L, Stevens MM (2018) A serological point-of-care test for the detection of igg antibodies against ebola virus in human survivors. ACS Nano 12(1):63–73. https://doi.org/10.1021/acsnano.7b07021
Bonacchini GE, Bossio C, Greco F, Mattoli V, Kim YH, Lanzani G, Caironi M (2018) Tattoo-paper transfer as a versatile platform for all-printed organic edible electronics. Adv Mater 30(14):e1706091. https://doi.org/10.1002/adma.201706091
Nilghaz A, Guan LY, Tan WR, Shen W (2016) Advances of paper-based microfluidics for diagnostics—the original motivation and current status. ACS Sensors 1(12):1382–1393. https://doi.org/10.1021/acssensors.6b00578
Luo L, Li X, Crooks RM (2014) Low-voltage origami-paper-based electrophoretic device for rapid protein separation. Anal Chem 86(24):12390–12397. https://doi.org/10.1021/ac503976c
Gan W, Gu Y, Han J, Li CX, Sun J, Liu P (2017) Chitosan-modified filter paper for nucleic acid extraction and "in Situ PCR" on a thermoplastic microchip. Anal Chem 89(6):3568–3575. https://doi.org/10.1021/acs.analchem.6b04882
Tiwari S, Vinchurkar M, Rao VR, Garnier G (2017) Zinc oxide nanorods functionalized paper for protein preconcentration in biodiagnostics. Sci Rep 7:43905. https://doi.org/10.1038/srep43905
Choi JR, Hu J, Feng S, Wan Abas WA, Pingguan-Murphy B, Xu F (2016) Sensitive biomolecule detection in lateral flow assay with a portable temperature-humidity control device. Biosens Bioelectron 79:98–107. https://doi.org/10.1016/j.bios.2015.12.005
Tang RH, Yang H, Gong Y, You ML, Liu Z, Choi JR, Wen T, Qu ZG, Mei QB, Xu F (2017) A fully disposable and integrated paper-based device for nucleic acid extraction, amplification and detection. Lab Chip 17(7):1270–1279. https://doi.org/10.1039/c6lc01586g
Lopez Marzo AM, Pons J, Blake DA, Merkoci A (2013) All-integrated and highly sensitive paper based device with sample treatment platform for Cd2+ immunodetection in drinking/tap waters. Anal Chem 85(7):3532–3538. https://doi.org/10.1021/ac3034536
Yang XX, Forouzan O, Brown TP, Shevkoplyas SS (2012) Integrated separation of blood plasma from whole blood for microfluidic paper-based analytical devices. Lab Chip 12:274–280. https://doi.org/10.1039/c1lc20803a
McFall SM, Wagner RL, Jangam SR, Yamada DH, Hardie D, Kelso DM (2015) A simple and rapid DNA extraction method from whole blood for highly sensitive detection and quantitation of HIV-1 proviral DNA by real-time PCR. J Virol Methods 214:37–42. https://doi.org/10.1016/j.jviromet.2015.01.005
Zhang Y, Rojas OJ (2017) Immunosensors for C-reactive protein based on ultrathin films of carboxylated cellulose nanofibrils. Biomacromolecules 18(2):526–534. https://doi.org/10.1021/acs.biomac.6b01681
Liu J, He Y, Chen S, Ma M, Yao S, Chen B (2017) New urea-modified paper substrate for enhanced analytical performance of negative ion mode paper spray mass spectrometry. Talanta 166:306–314. https://doi.org/10.1016/j.talanta.2017.01.076
Tang RH, Yang H, Choi JR, Gong Y, Hu J, Wen T, Li XJ, Xu B, Mei QB, Xu F (2017) Paper-based device with on-chip reagent storage for rapid extraction of DNA from biological samples. Microchim Acta 184(7):2141–2150. https://doi.org/10.1007/s00604-017-2225-0
Songjaroen T, Dungchai W, Chailapakul O, Henry CS, Laiwattanapaisal W (2012) Blood separation on microfluidic paper-based analytical devices. Lab Chip 12(18):3392–3398. https://doi.org/10.1039/c2lc21299d
Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH (2013) Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Anal Chem 85(21):10463–10470. https://doi.org/10.1021/ac402459h
Mosley GL, Pereira DY, Han Y, Lee SY, Wu CM, Wu BM, Kamei DT (2017) Improved lateral-flow immunoassays for chlamydia and immunoglobulin M by sequential rehydration of two-phase system components within a paper-based diagnostic. Microchim Acta 184(10):4055–4064. https://doi.org/10.1007/s00604-017-2434-6
Lu X, Mei T, Guo Q, Zhou W, Li X, Chen J, Zhou X, Sun N, Fang Z (2018) Improved performance of lateral flow immunoassays for alpha-fetoprotein and vanillin by using silica shell-stabilized gold nanoparticles. Mikrochimica Acta 186(1):2. https://doi.org/10.1007/s00604-018-3107-9
Derikvand F, Yin DT, Barrett R, Brumer H (2016) Cellulose-Based Biosensors for Esterase Detection. Anal Chem 88(6):2989–2993. https://doi.org/10.1021/acs.analchem.5b04661
Yan JX, Yan M, Ge L, Ge SG, Yu JH (2014) An origami electrochemiluminescence immunosensor based on gold/graphene for specific, sensitive point-of-care testing of carcinoembryonic antigen. Sensors Actuators B Chem 193:247–254. https://doi.org/10.1016/j.snb.2013.11.107
Li ZD, Li F, Xing Y, Liu Z, You ML, Li YC, Wen T, Qu ZG, Li XL, Xu F (2017) Pen-on-paper strategy for point-of-care testing: rapid prototyping of fully written microfluidic biosensor. Biosens Bioelectron 98:478–485. https://doi.org/10.1016/j.bios.2017.06.061
Butwong N, Kunthadong P, Soisungnoen P, Chotichayapong C, Srijaranai S, Luong JHT (2018) Silver-doped CdS quantum dots incorporated into chitosan-coated cellulose as a colorimetric paper test stripe for mercury. Mikrochimica Acta 185(2):126. https://doi.org/10.1007/s00604-018-2671-3
Anfossi L, Di Nardo F, Cavalera S, Giovannoli C, Spano G, Speranskaya ES, Goryacheva IY, Baggiani C (2018) A lateral flow immunoassay for straightforward determination of fumonisin mycotoxins based on the quenching of the fluorescence of CdSe/ZnS quantum dots by gold and silver nanoparticles. Mikrochimica Acta 185(2):94. https://doi.org/10.1007/s00604-017-2642-0
Sheng W, Chang Q, Shi Y, Duan W, Zhang Y, Wang S (2018) Visual and fluorometric lateral flow immunoassay combined with a dual-functional test mode for rapid determination of tetracycline antibiotics. Mikrochimica Acta 185(9):404. https://doi.org/10.1007/s00604-018-2945-9
Sheng W, Li S, Liu Y, Wang J, Zhang Y, Wang S (2017) Visual and rapid lateral flow immunochromatographic assay for enrofloxacin using dyed polymer microspheres and quantum dots. Microchim Acta 184(11):4313–4321. https://doi.org/10.1007/s00604-017-2474-y
Nutthaya Butwong PK, Soisungnoen P, Chotichiayapong C, Srijaranai S, Luong JHT (2018) Silver-doped CdS quantum dots incorporated into chitosan-coated cellulose as a colorimetric paper test stripe for mercury. Microchim Acta 185:126–135
Magiati M, Sevastou A, Kalogianni DP (2018) A fluorometric lateral flow assay for visual detection of nucleic acids using a digital camera readout. Mikrochimica Acta 185(6):314. https://doi.org/10.1007/s00604-018-2856-9
Boonkaew S, Chaiyo S, Jampasa S, Rengpipat S, Siangproh W, Chailapakul O (2019) An origami paper-based electrochemical immunoassay for the C-reactive protein using a screen-printed carbon electrode modified with graphene and gold nanoparticles. Mikrochimica Acta 186(3):153. https://doi.org/10.1007/s00604-019-3245-8
Taranova NA, Urusov AE, Sadykhov EG, Zherdev AV, Dzantiev BB (2017) Bifunctional gold nanoparticles as an agglomeration-enhancing tool for highly sensitive lateral flow tests: a case study with procalcitonin. Microchim Acta 184(10):4189–4195. https://doi.org/10.1007/s00604-017-2355-4
Wang W, Liu L, Song S, Xu L, Kuang H, Zhu J, Xu C (2016) Identification and quantification of eight Listeria monocytogene serotypes from Listeria spp. using a gold nanoparticle-based lateral flow assay. Microchim Acta 184(3):715–724. https://doi.org/10.1007/s00604-016-2028-8
Ruppert C, Phogat N, Laufer S, Kohl M, Deigner HP (2019) A smartphone readout system for gold nanoparticle-based lateral flow assays: application to monitoring of digoxigenin. Mikrochimica Acta 186(2):119. https://doi.org/10.1007/s00604-018-3195-6
Shi QQ, Huang J, Sun YN, Deng RG, Teng M, Li QM, Yang YY, Hu XF, Zhang ZJ, Zhang GP (2018) A SERS-based multiple immuno-nanoprobe for ultrasensitive detection of neomycin and quinolone antibiotics via a lateral flow assay. Microchem J 185(2):84–92. https://doi.org/10.1007/s00604-017-2556-x
Yao L, Teng J, Qu H, Zhu M, Zheng L, Xue F, Chen W (2016) Paper matrix based array for rapid and sensitive optical detection of mercury ions using silver enhancement. Microchim Acta 184(2):569–576. https://doi.org/10.1007/s00604-016-2052-8
Li S, Wang J, Sheng W, Wen W, Gu Y, Wang S (2018) Fluorometric lateral flow immunochromatographic zearalenone assay by exploiting a quencher system composed of carbon dots and silver nanoparticles. Microchim Acta 185(8). https://doi.org/10.1007/s00604-018-2916-1
Urusov AE, Gubaidullina MK, Petrakova AV, Zherdev AV, Dzantiev BB (2018) A new kind of highly sensitive competitive lateral flow immunoassay displaying direct analyte-signal dependence. Application to the determination of the mycotoxin deoxynivalenol. Mikrochimica Acta 185(1):29. https://doi.org/10.1007/s00604-017-2576-6
Panferov VG, Safenkova IV, Varitsev YA, Zherdev AV, Dzantiev BB (2017) Enhancement of lateral flow immunoassay by alkaline phosphatase: a simple and highly sensitive test for potato virus X. Mikrochimica Acta 185(1):25. https://doi.org/10.1007/s00604-017-2595-3
Cheng T, Zhang Y, Liu X, Zhang X, Zhang H (2017) A filter paper coated with phenylboronic acid-modified mesoporous silica for enrichment of intracellular nucleosides prior to their quantitation by HPLC. Microchim Acta 184(10):4007–4013. https://doi.org/10.1007/s00604-017-2423-9
Pungjunun K, Chaiyo S, Jantrahong I, Nantaphol S, Siangproh W, Chailapakul O (2018) Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Mikrochimica Acta 185(7):324. https://doi.org/10.1007/s00604-018-2821-7
Faham S, Khayatian G, Golmohammadi H, Ghavami R (2018) A paper-based optical probe for chromium by using gold nanoparticles modified with 2,2'-thiodiacetic acid and smartphone camera readout. Mikrochimica Acta 185(8):374. https://doi.org/10.1007/s00604-018-2875-6
Fu X, Chu Y, Zhao K, Li J, Deng A (2017) Ultrasensitive detection of the β-adrenergic agonist brombuterol by a SERS-based lateral flow immunochromatographic assay using flower-like gold-silver core-shell nanoparticles. Microchim Acta 184(6):1711–1719. https://doi.org/10.1007/s00604-017-2178-3
Darabdhara G, Boruah PK, Das MR (2018) Colorimetric determination of glucose in solution and via the use of a paper strip by exploiting the peroxidase and oxidase mimicking activity of bimetallic Cu-Pd nanoparticles deposited on reduced graphene oxide, graphitic carbon nitride, or MoS2 nanosheets. Mikrochimica Acta 186(1):13. https://doi.org/10.1007/s00604-018-3112-z
Zhang Y, Liao Z, Liu Y, Wan Y, Chang J, Wang H (2017) Flow cytometric immunoassay for aflatoxin B1 using magnetic microspheres encoded with upconverting fluorescent nanocrystals. Microchim Acta 184(5):1471–1479. https://doi.org/10.1007/s00604-017-2116-4
Ortiz-Gomez I, Salinas-Castillo A, Garcia AG, Alvarez-Bermejo JA, de Orbe-Paya I, Rodriguez-Dieguez A, Capitan-Vallvey LF (2017) Microfluidic paper-based device for colorimetric determination of glucose based on a metal-organic framework acting as peroxidase mimetic. Mikrochimica Acta 185(1):47. https://doi.org/10.1007/s00604-017-2575-7
Wang R, Zhang W, Wang P, Su X (2018) A paper-based competitive lateral flow immunoassay for multi beta-agonist residues by using a single monoclonal antibody labelled with red fluorescent nanoparticles. Mikrochimica Acta 185(3):191. https://doi.org/10.1007/s00604-018-2730-9
Yang Y, Ozsoz M, Liu G (2017) Gold nanocage-based lateral flow immunoassay for immunoglobulin G. Mikrochimica Acta 184(7):2023–2029. https://doi.org/10.1007/s00604-017-2176-5
Tao Y, Yang J, Chen L, Huang Y, Qiu B, Guo L, Lin Z (2018) Dialysis assisted ligand exchange on gold nanorods: Amplification of the performance of a lateral flow immunoassay for E. coli O157:H7. Mikrochimica Acta 185(7):350. https://doi.org/10.1007/s00604-018-2897-0
Weng X, Neethirajan S (2017) Aptamer-based fluorometric determination of norovirus using a paper-based microfluidic device. Microchim Acta 184(11):4545–4552. https://doi.org/10.1007/s00604-017-2467-x
Wang Y, Wang Y, Li D, Xu J, Ye C (2018) Detection of nucleic acids and elimination of carryover contamination by using loop-mediated isothermal amplification and antarctic thermal sensitive uracil-DNA-glycosylase in a lateral flow biosensor: application to the detection of Streptococcus pneumoniae. Microchim Acta 185(4). https://doi.org/10.1007/s00604-018-2723-8
Hu J, Wang SQ, Wang L, Li F, Pingguan-Murphy B, Lu TJ, Xu F (2014) Advances in paper-based point-of-care diagnostics. Biosens Bioelectron 54:585–597. https://doi.org/10.1016/j.bios.2013.10.075
Gong MM, Sinton D (2017) Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem Rev 117(12):8447–8480. https://doi.org/10.1021/acs.chemrev.7b00024
Yamada K, Henares TG, Suzuki K, Citterio D (2015) Paper-based inkjet-printed microfluidic analytical device. Angew Chem Int Ed 54:2–19. https://doi.org/10.1002/anie.201411508
Xu Y, Liu M, Kong N, Liu J (2016) Lab-on-paper micro- and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim Acta 183(5):1521–1542. https://doi.org/10.1007/s00604-016-1841-4
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem 46(8):1318–1320. https://doi.org/10.1002/anie.200603817
Sahin HT, Arslan MB (2008) A study on physical and chemical properties of cellulose paper immersed in various solvent mixtures. Int J Mol Sci 9:78–88. https://doi.org/10.3390/ijms9010078
Shen J, Song ZQ, Qian XR, Liu WX (2009) Modification of papermaking grade fillers: a brief review. BioResources 4(3):1190–1209. https://doi.org/10.1007/s00226-008-0200-y
Elizalde E, Urteaga R, Berli CLA (2016) Precise capillary flow for paper-based viscometry. Microfluid Nanofluid 20(10). https://doi.org/10.1007/s10404-016-1800-8
Tang RH, Yang H, Gong Y, Liu Z, Li X, Wen T, Qu Z, Zhang S, Mei QB, Xu F (2017) Improved analytical sensitivity of lateral flow assay using sponge for HBV nucleic acid detection. Sci Rep 7(1):1360. https://doi.org/10.1038/s41598-017-01558-x
Jeong S-G, Kim J, Jin SH, Park K-S, Lee C-S (2016) Flow control in paper-based microfluidic device for automatic multistep assays: a focused minireview. Korean J Chem Eng 33(10):2761–2770. https://doi.org/10.1007/s11814-016-0161-z
Ballerini DR, Li X, Shen W (2012) Patterned paper and alternative materials as substrates for low-cost microfluidic diagnostics. Microfluid Nanofluid 13(5):769–787. https://doi.org/10.1007/s10404-012-0999-2
Mohite BV, Patil SV (2014) Bacterial cellulose of Gluconoacetobacter hansenii as a potential bioadsorption agent for its green environment applications. J Biomater Sci Polym Ed 25(18):2053–2065. https://doi.org/10.1080/09205063.2014.970063
Wu Y, Luo H, Wang H, Zhang L, Liu P, Feng L (2014) Fast adsorption of nickel ions by porous graphene oxide/sawdust composite and reuse for phenol degradation from aqueous solutions. J Colloid Interface Sci 436:90–98. https://doi.org/10.1016/j.jcis.2014.08.068
Wei X, Huang T, Yang JH, Zhang N, Wang Y, Zhou ZW (2017) Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J Hazard Mater 335:28–38. https://doi.org/10.1016/j.jhazmat.2017.04.030
Zhou Y, Min Y, Qiao H, Huang Q, Wang E, Ma T (2015) Improved removal of malachite green from aqueous solution using chemically modified cellulose by anhydride. Int J Biol Macromol 74:271–277. https://doi.org/10.1016/j.ijbiomac.2014.12.020
Zhang Y, Liu Y, Wang X, Sun Z, Ma J, Wu T, Xing F, Gao J (2014) Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr Polym 101:392–400. https://doi.org/10.1016/j.carbpol.2013.09.066
Li Q, Sun J, Ren T, Guo L, Yang Z, Yang Q, Chen H (2018) Adsorption mechanism of 2,4-dichlorophenoxyacetic acid onto nitric-acid-modified activated carbon fiber. Environ Technol 39(7):895–906. https://doi.org/10.1080/09593330.2017.1316318
Deng H, Ning J, Wang X (2016) Amino-functionalized cotton fiber for enhanced adsorption of active brilliant red X-3B from aqueous solution. Microsc Res Tech 79(12):1200–1207. https://doi.org/10.1002/jemt.22778
Li J, Liao XP, Zhang QX, Shi B (2013) Adsorption and separation of proteins by collagen fiber adsorbent. J Chromatogr B Anal Technol Biomed Life Sci 928:131–138. https://doi.org/10.1016/j.jchromb.2013.03.031
Lu T, Chen X, Shi Q, Wang Y, Zhang P, Jing X (2008) The immobilization of proteins on biodegradable fibers via biotin-streptavidin bridges. Acta Biomater 4(6):1770–1777. https://doi.org/10.1016/j.actbio.2008.05.006
Serafica GC, Pimbley J, Belfort G (1994) Protein fractionation using fast flow immobilized metal chelate affinity membranes. Biotechnol Bioeng 43:21–36. https://doi.org/10.1002/bit.260430105
Lowa SC, Shaimi R, Thandaithabany Y, Lim JK, Ahmad AL, Ismail A (2013) Electrophoretic interactions between nitrocellulose membranes and proteins: Biointerface analysis and protein adhesion properties. Colloids Surf B: Biointerfaces 110:248–253. https://doi.org/10.1016/j.colsurfb.2013.05.001
Sekhar CP, Kalidhasan S, Rajesh V, Rajesh N (2009) Bio-polymer adsorbent for the removal of malachite green from aqueous solution. Chemosphere 77(6):842–847. https://doi.org/10.1016/j.chemosphere.2009.07.068
Chang F, Xue S, Xie X, Fang W, Fang Z, Xiao Y (2018) Carbohydrate-binding module assisted purification and immobilization of beta-glucosidase onto cellulose and application in hydrolysis of soybean isoflavone glycosides. J Biosci Bioeng 125(2):185–191. https://doi.org/10.1016/j.jbiosc.2017.09.001
Kono H, Ogasawara K, Kusumoto R, Oshima K, Hashimoto H, Shimizu Y (2016) Cationic cellulose hydrogels cross-linked by poly(ethylene glycol): Preparation, molecular dynamics, and adsorption of anionic dyes. Carbohyd Polym 152:170–180. https://doi.org/10.1016/j.carbpol.2016.07.011
Saravanan R, Ravikumar L (2016) Cellulose bearing Schiff base and carboxylic acid chelating groups: a low cost and green adsorbent for heavy metal ion removal from aqueous solution. Water science and technology : a journal of the International Association on Water Pollution Research 74(8):1780–1792. https://doi.org/10.2166/wst.2016.296
Song T-T, Wang W, Meng L-L, Liu Y, Jia X-B, Mao X (2017) Electrochemical detection of human ferritin based on gold nanorod reporter probe and cotton thread immunoassay device. Chin Chem Lett 28(2):226–230. https://doi.org/10.1016/j.cclet.2016.07.021
Meng LL, Song TT, Mao X (2017) Novel immunochromatographic assay on cotton thread based on carbon nanotubes reporter probe. Talanta 167:379–384. https://doi.org/10.1016/j.talanta.2017.02.023
Xun M, Du T-E, Yiyun W, Lili M (2015) Disposable dry-reagent cotton thread-based point-of-care diagnosis device for protein and nucleic acid test. Biosens Bioelectron 65:390–396. https://doi.org/10.1016/j.bios.2014.10.053
Du T-E, Yiyun W, Yi Z, Tian Z, Xun M (2015) A novel adenosine-based molecular beacon probe for room temperature nucleic acid rapid detection in cotton thread device. Anal Chim Acta 861:69–73. https://doi.org/10.1016/j.aca.2014.12.044
Ummartyotin S, Manuspiya H (2015) A critical review on cellulose: From fundamental to an approach on sensor technology. Renew Sust Energ Rev 41:402–412. https://doi.org/10.1016/j.rser.2014.08.050
Suhas GVK, Carrott PJ, Singh R, Chaudhary M, Kushwaha S (2016) Cellulose: A review as natural, modified and activated carbon adsorbent. Bioresour Technol 216:1066–1076. https://doi.org/10.1016/j.biortech.2016.05.106
Thakur VK, Thakur MK (2014) Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr Polym 109:102–117. https://doi.org/10.1016/j.carbpol.2014.03.039
Hu W, Chen S, Yang J, Li Z, Wang H (2014) Functionalized bacterial cellulose derivatives and nanocomposites. Carbohydr Polym 101:1043–1060. https://doi.org/10.1016/j.carbpol.2013.09.102
Petersen N, Gatenholm P (2011) Bacterial cellulose-based materials and medical devices: current state and perspectives. Appl Microbiol Biotechnol 91(5):1277–1286. https://doi.org/10.1007/s00253-011-3432-y
Mohite BV, Patil SV (2014) Physical, structural, mechanical and thermal characterization of bacterial cellulose by G. hansenii NCIM 2529. Carbohydr Polym 106:132–141. https://doi.org/10.1016/j.carbpol.2014.02.012
Tang WH, Jia SR, Jia YY, Yang HJ (2009) The influence of fermentation conditions and post-treatment methods on porosity of bacterial cellulose membrane. World J Microbiol Biotechnol 26(1):125–131. https://doi.org/10.1007/s11274-009-0151-y
El-Zawahry MM, Abdelghaffar F, Abdelghaffar RA, Hassabo AG (2016) Equilibrium and kinetic models on the adsorption of Reactive Black 5 from aqueous solution using Eichhornia crassipes/chitosan composite. Carbohydr Polym 136:507–515. https://doi.org/10.1016/j.carbpol.2015.09.071
Jungmi Lee AASS, Yim Y, Kim S-y, Jeon NL, Min D-H (2019) A FRET assay for the quantitation of inhibitors of exonuclease EcoRV by using parchment paper inkjet-printed with graphene oxide and FAM-labelled DNA. Microchim Acta 186:211–221
Deák T, Czigány T (2009) Chemical composition and mechanical properties of basalt and glass fibers: a comparison. Text Res J 79(7):645–651. https://doi.org/10.1177/0040517508095597
Hejazi SM, Sheikhzadeh M, Abtahi SM, Zadhoush A (2012) A simple review of soil reinforcement by using natural and synthetic fibers. Constr Build Mater 30:100–116. https://doi.org/10.1016/j.conbuildmat.2011.11.045
Velmurugan R, Manikandan V (2007) Mechanical properties of palmyra/glass fiber hybrid composites. Compos A: Appl Sci Manuf 38(10):2216–2226. https://doi.org/10.1016/j.compositesa.2007.06.006
Fridley GE, Holstein CA, Oza SB, Yager P (2013) The evolution of nitrocellulose as a material for bioassays. MRS Bull 38(04):326–330. https://doi.org/10.1557/mrs.2013.60
B Kabariya R, M Ramani V (2018) Exploration of thread for their possible use in fabrication of low cost diagnostic microfluidic device. Mater Sci Res India 15(2):179–184. https://doi.org/10.13005/msri/150210
Nilghaz A, Ballerini DR, Shen W (2013) Exploration of microfluidic devices based on multi-filament threads and textiles: a review. Biomicrofluidic 7(5):0051501–0051516. https://doi.org/10.1063/1.4820413
Gong Y, Hu J, Choi JR, You ML, Zheng YM, Xu B, Wen T, Xu F (2017) Improved LFIAs for highly sensitive detection of BNP at point-of-care. Int J Nanomedicine Volume 12:4455-4466. doi:https://doi.org/10.2147/ijn.s135735
Tang RH, Yang H, Choi JR, Gong Y, Hu J, Feng SS, Pingguan-Murphy B, Mei QB, Xu F (2016) Improved sensitivity of lateral flow assay using paper-based sample concentration technique. Talanta 152:269–276. https://doi.org/10.1016/j.talanta.2016.02.017
Rivas L, Medina-Sanchez M, de la Escosura-Muniz A, Merkoci A (2014) Improving sensitivity of gold nanoparticle-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics. Lab Chip 14(22):4406–4414. https://doi.org/10.1039/c4lc00972j
Lonnberg M, Carlsson J (2001) Quantitative detection in the attomole range for immunochromatographic tests by means of a flatbed scanner. Anal Biochem 293(2):224–231. https://doi.org/10.1006/abio.2001.5130
Wang Y, Wang L, Zhang C, Liu F (2019) A lateral flow assay for copper(II) utilizing catalytic and stem-loop based signal amplification. Mikrochimica Acta 186(2):82. https://doi.org/10.1007/s00604-018-3197-4
Zhang J, Lv X, Feng W, Li X, Li K, Deng Y (2018) Aptamer-based fluorometric lateral flow assay for creatine kinase MB. Mikrochimica Acta 185(8):364. https://doi.org/10.1007/s00604-018-2905-4
Hu J, Wang L, Li F, Han YL, Lin M, Lu TJ, Xu F (2013) Oligonucleotide-linked gold nanoparticle aggregates for enhanced sensitivity in lateral flow assays. Lab Chip 13(22):4352–4357. https://doi.org/10.1039/c3lc50672j
Araujo AC, Song Y, Lundeberg J, Stahl PL, Brumer H (2012) Activated paper surfaces for the rapid hybridization of DNA through capillary transport. Anal Chem 84(7):3311–3317. https://doi.org/10.1021/ac300025v
Misteli T, Zou YP, Mason MG, Wang YL, Wee E, Turni C, Blackall PJ, Trau M, Botella JR (2017) Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol 15(11):e2003916. https://doi.org/10.1371/journal.pbio.2003916
Kun-Hua Lu J-HL, LIn C-Y, Chen C-F, Yeh Y-C (2019) A fluorometric paper test for chromium(VI) based on the use of N-doped carbon dots. Microchim Acta 186:227–234
Shi-jie Li WS, Wen W, Ying G, Wang J-p, Wang S (2018) Three kinds of lateral flow immunochromatographic assays based on the use of nanoparticle labels for fluorometric determination of zearalenone. Microchim Acta 185:238–246
Panferov VG, Safenkova IV, Zherdev AV, Dzantiev BB (2018) Post-assay growth of gold nanoparticles as a tool for highly sensitive lateral flow immunoassay. Application to the detection of potato virus X. Mikrochimica Acta 185(11):506. https://doi.org/10.1007/s00604-018-3052-7
Juanzu Liu JW, Li Z, Meng H, Zhang L, Wang H, Li J, Lingbo Q (2018) A lateral flow assay for the determination of human tetanus antibody in whole blood by using gold nanoparticle labeled tetanus antigen. Microchim Acta 185:110–116
Choi JR, Hu J, Tang R, Gong Y, Feng S, Ren H, Wen T, Li X, Wan Abas WA, Pingguan-Murphy B, Xu F (2016) An integrated paper-based sample-to-answer biosensor for nucleic acid testing at the point of care. Lab Chip 16(3):611–621. https://doi.org/10.1039/c5lc01388g
Jones S, Sutherland CJ, Hermsen C, Arens T, Teelen K, Hallett R, Corran P, van der Vegte-Bolmer M, Sauerwein R, Drakeley CJ, Bousema T (2012) Filter paper collection of Plasmodium falciparum mRNA for detecting low-density gametocytes. Malar J 11:266-275. doi:https://doi.org/10.1186/1475-2875-11-266
Dusek RJ, Hall JS, Nashold SW, TeSlaa JL, Ip HS (2011) Evaluation of Nobuto filter paper strips for the detection of avian Antibody in Waterfowl. Avian Dis 55:674–676. https://doi.org/10.1637/9687-021511-ResNote.1
Pritsch M, Wieser A, Soederstroem V, Poluda D, Eshetu T, Hoelscher M, Schubert S, Shock J, Loescher T, Berens-Riha N (2012) Stability of gametocyte-specific Pfs25-mRNA in dried blood spots on filter paper subjected to different storage conditions. Malar J 11:138–144. https://doi.org/10.1186/1475-2875-11-138
Metherel AH, Aristizabal Henao JJ, Stark KD (2013) EPA and DHA levels in whole blood decrease more rapidly when stored at -20 degrees C as compared with room temperature, 4 and -75 degrees C. Lipids 48(11):1079–1091. https://doi.org/10.1007/s11745-013-3827-x
Ulum MF, Maylina L, Noviana D, Wicaksono DH (2016) EDTA-treated cotton-thread microfluidic device used for one-step whole blood plasma separation and assay. Lab Chip 16(8):1492–1504. https://doi.org/10.1039/c6lc00175k
Kraus RH, van Hooft P, Waldenstrom J, Latorre-Margalef N, Ydenberg RC, Prins HH (2011) Avian influenza surveillance with FTA cards: field methods, biosafety, and transportation issues solved. Jove-J Vis Exp (54). https://doi.org/10.3791/2832
Maignan M, Viglino D, Hablot M, Termoz Masson N, Lebeugle A, Collomb Muret R, Mabiala Makele P, Guglielmetti V, Morand P, Lupo J, Forget V, Landelle C, Larrat S (2019) Diagnostic accuracy of a rapid RT-PCR assay for point-of-care detection of influenza A/B virus at emergency department admission: a prospective evaluation during the 2017/2018 influenza season. PLoS One 14(5):e0216308. https://doi.org/10.1371/journal.pone.0216308
Yang C-H, Chen C-A, Chen C-F (2018) Surface-modified cellulose paper and its application in infectious disease diagnosis. Sensors Actuators B Chem 265:506–513. https://doi.org/10.1016/j.snb.2018.03.092
Zhang H, Lei Z, Tian R, Wang Z (2018) Polyamidoamine starburst dendrimer-activated chromatography paper-based assay for sensitive detection of telomerase activity. Talanta 178:116–121. https://doi.org/10.1016/j.talanta.2017.09.034
Moazeni M, Karimzadeh F, Kermanpur A (2018) Peptide modified paper based impedimetric immunoassay with nanocomposite electrodes as a point-of-care testing of Alpha-fetoprotein in human serum. Biosens Bioelectron 117:748–757. https://doi.org/10.1016/j.bios.2018.07.016
Chan SK, Lim TS (2016) A straw-housed paper-based colorimetric antibody–antigen sensor. Anal Methods 8(6):1431–1436. https://doi.org/10.1039/c5ay01828e
Wang S, Ge L, Song X, Yu J, Ge S, Huang J, Zeng F (2012) Paper-based chemiluminescence ELISA: lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron 31(1):212–218. https://doi.org/10.1016/j.bios.2011.10.019
Li W, Ge S, Wang S, Yan M, Ge L, Yu J (2013) Highly sensitive chemiluminescence immunoassay on chitosan membrane modified paper platform using TiO2 nanoparticles/multiwalled carbon nanotubes as label. Luminescence 28(4):496–502. https://doi.org/10.1002/bio.2482
Marín-Barroso E, Moreira CM, Messina GA, Bertolino FA, Alderete M, Soler-Illia GJAA, Raba J, Pereira SV (2018) Paper based analytical device modified with nanoporous material for the fluorescent sensing of gliadin content in different food samples. Microchem J 142:78–84. https://doi.org/10.1016/j.microc.2018.06.005
Choi JR, Nilghaz A, Chen L, Chou KC, Lu X (2018) Modification of thread-based microfluidic device with polysiloxanes for the development of a sensitive and selective immunoassay. Sensors Actuators B Chem 260:1043–1051. https://doi.org/10.1016/j.snb.2018.01.102
Preechakasedkit P, Siangproh W, Khongchareonporn N, Ngamrojanavanich N, Chailapakul O (2018) Development of an automated wax-printed paper-based lateral flow device for alpha-fetoprotein enzyme-linked immunosorbent assay. Biosens Bioelectron 102:27–32. https://doi.org/10.1016/j.bios.2017.10.051
Lu JJ, Ge SG, Ge L, Yan M, Yu JH (2012) Electrochemical DNA sensor based on three-dimensional folding paper device for specific and sensitive point-of-care testing. Electrochim Acta 80:334–341. https://doi.org/10.1016/j.electacta.2012.07.024
Gomes HI, Sales MG (2015) Development of paper-based color test-strip for drug detection in aquatic environment: Application to oxytetracycline. Biosens Bioelectron 65:54–61. https://doi.org/10.1016/j.bios.2014.10.006
Wang Y, Luo J, Liu J, Li X, Kong Z, Jin H, Cai X (2018) Electrochemical integrated paper-based immunosensor modified with multi-walled carbon nanotubes nanocomposites for point-of-care testing of 17beta-estradiol. Biosens Bioelectron 107:47–53. https://doi.org/10.1016/j.bios.2018.02.012
Devadhasan JP, Kim J (2018) A chemically functionalized paper-based microfluidic platform for multiplex heavy metal detection. Sensors Actuators B Chem 273:18–24. https://doi.org/10.1016/j.snb.2018.06.005
Cao R, Zhang X, Tan W, Shen W (2018) Precipitation assay meets low wettability on paper: a simple approach for fabricating patterned paper sensors. Cellulose 25(1):583–592. https://doi.org/10.1007/s10570-017-1551-z
Chaiyo S, Mehmeti E, Siangproh W, Hoang TL, Nguyen HP, Chailapakul O, Kalcher K (2018) Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine-ionic liquid-graphene composite. Biosens Bioelectron 102:113–120. https://doi.org/10.1016/j.bios.2017.11.015
Salekin S, Bari MG, Raphael I, Forsthuber TG, Zhang JM (2017) Early disease correlated protein detection using early response index (ERI). BMC Bioinformatics 18:313–324
Shafiee H, Asghar W, Inci F, Yuksekkaya M, Jahangir M, Zhang MH, Durmus NG, Gurkan UA, Kuritzkes DR, Demirci U (2015) Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Sci Rep 5:8719. https://doi.org/10.1038/srep08719
Liu Z, Hu J, Li A, Feng SS, Qu ZG, Xu F (2017) The effect of report particle properties on lateral flow assays: A mathematical model. Sensors Actuators B Chem 248:699–707. https://doi.org/10.1016/j.snb.2017.04.024
Liu Z, Hu J, Zhao YM, Qu ZG, Xu F (2015) Experimental and numerical studies on liquid wicking into filter papers for paper-based diagnostics. Appl Therm Eng 88:280–287. https://doi.org/10.1016/j.applthermaleng.2014.09.057
Wang W, Liang T, Bai H, Dong W, Liu X (2018) All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohydr Polym 179:297–304. https://doi.org/10.1016/j.carbpol.2017.09.098
Natalio D, Fuchs R, Cohen SR, Leitus G, Fritz-Popovski G, Paris O, Kappi M, Butt H-J (2017) Biological fabrication of cellulose fibers with tailored properties. Science 357:1118–1122. https://doi.org/10.1126/science.aan5830
Lee CH, Chuang WY, Cowan MA, Wu WJ, Lin CT (2014) A low-power integrated humidity CMOS sensor by printing-on-chip technology. Sensors 14(5):9247–9255. https://doi.org/10.3390/s140509247
Bhakta SA, Borba R, Taba M, Jr., Garcia CD, Carrilho E (2014) Determination of nitrite in saliva using microfluidic paper-based analytical devices. Anal Chim Acta 809:117-122. doi:https://doi.org/10.1016/j.aca.2013.11.044
Cate DM, Dungchai W, Cunningham JC, Volckens J, Henry CS (2013) Simple, distance-based measurement for paper analytical devices. Lab Chip 13(12):2397–2404. https://doi.org/10.1039/c3lc50072a
Rahman Bhuiyan MA, Hossain MA, Zakaria M, Islam MN, Zulhash Uddin M (2016) Chitosan Coated Cotton Fiber: Physical and Antimicrobial Properties for Apparel Use. J Polym Environ 25(2):334–342. https://doi.org/10.1007/s10924-016-0815-2
Son SU, Seo SB, Jang S, Choi J, J-w L, Lee DK, Kim H, Seo S, Kang T, Jung J, Lim E-K (2019) Naked-eye detection of pandemic influenza a (pH1N1) virus by polydiacetylene (PDA)-based paper sensor as a point-of-care diagnostic platform. Sensors Actuators B Chem 291:257–265. https://doi.org/10.1016/j.snb.2019.04.081
Fan Y, Shi S, Ma J, Guo Y (2019) A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125. Biosens Bioelectron 135:1–7. https://doi.org/10.1016/j.bios.2019.03.063
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21808132, 31700510, 21774071), the China Postdoctoral Science Foundation (2018M633525), the Project of Shaanxi Provincial Education department (18JK0096), the Natural Science Basic Research Plan of Shaanxi Province (2019JQ-517), the Opening Project of National Experimental Teaching Demonstration Center of Light Chemical Engineering (2018QGSJ02-10), the High-level Foreign Experts Project (GD20186100425), the Natural Science Research Foundation of Shaanxi University of Science & Technology (2017BJ-35), “Biomass Chemistry and Materials” Academician Workstation Project in SUST (134090002), Key Scientific Research Group of Shaanxi Province (2017KCT-02) and the Key Program for Science and Technology Innovative Research Team in Shaanxi Province of China (2017KCT-22).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, R.H., Liu, L.N., Zhang, S.F. et al. A review on advances in methods for modification of paper supports for use in point-of-care testing. Microchim Acta 186, 521 (2019). https://doi.org/10.1007/s00604-019-3626-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3626-z