Abstract
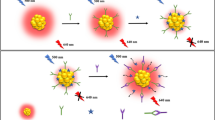
Glutathione-capped copper nanoclusters (CuNCs) are presented that display aggregation-induced emission (AIE). This feature was exploited for selective and sensitive quantification of creatinine (CRN) which is an important diagnostic parameter. In the presence of Al3+ ions, such CuNCs rapidly aggregate, and this induces enhanced a red emission. The AIE nature of CuNCs was proven via TEM and fluorimetry. On addition of CRN, the coordination between CRN and Al3+ ions led to the quenching of fluorescence due to weakening the AIE. The best fluorescence intensity was measured at excitation/emission peaks of 360/585 nm. Quenched fluorescence intensity showed a linear dependence on the concentrations of CRN in the range of 2.5–34 μgL−1 with a detection limit of 0.63 μgL−1. The sensing mechanism of probe for CRN detection is discussed. The probe was applied to the determination of CRN in spiked human serum samples and gave satisfactory results.

Aluminum(III) ions trigger the aggregation-induced emission (AIE) in glutathione-capped copper nanoclusters (CuNCs). Introduction of creatinine leads to deaggregation of CuNC aggregates, and this is accompanied by the quenching of the luminescence. This phenomenon was exploited in an assay for creatinine.





Similar content being viewed by others
References
Luo J, Xie Z, Lam JWY, Cheng L et al (2001) Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem Commun 381:1740–1741
Zhang L, He N, Lu C (2015) Aggregation-induced emission: a simple strategy to improve chemiluminescence resonance energy transfer. Anal Chem 87:1351–1357
Jia X, Yang X, Li J, Li D, Wang E (2014) Stable Cu nanoclusters: from an aggregation-induced emission mechanism to biosensing and catalytic applications. Chem Commun 50:237–239
Li H, Wang C, Hou T, Li F (2017) Amphiphile-mediated ultrasmall aiggregation induced emission dots for ultrasensitive fluorescence biosensing. Anal Chem 89:9100–9107
Shang L, Dong S, Nienhaus GU (2011) Ultra-small fluorescent metal nanoclusters: synthesis and biological applications. Nano Today 6:401–418
Chen YS, Kamat PV (2014) Glutathione capped gold nanoclusters as photosensitizers. Visible light induced hydrogen generation in neutral water. J Am Chem Soc 136:6075–6082
Yuan X, Tay Y, Dou X, Luo Z, Leong DT, Xie J (2013) Glutathione-protected silver nanoclusters as cysteine-selective fluorometric and colorimetric probe. Anal Chem 85:1913–1919
Wolfbeis OS (2015) An overview of nanoparticles commonly used in fluorescent bioimaging. Chem Soc Rev 44:4743–4768
Demir B, Lemberger M, Panagiotopoulou M, Paulina MR, Timur S, Hirsch T et al (2018) Tracking hyaluronan: molecularly imprinted polymer coated carbon dots for cancer cell targeting and imaging. ACS Appl Mater Interfaces 31:3305–3313
Amjadi M, Jalili R (2016) Molecularly imprinted polymer-capped nitrogen-doped graphene quantum dots as a novel chemiluminescence sensor for selective and sensitive determination of doxorubicin. RSC Adv 6:86736–86743
Amjadi M, Jalili R (2017) Molecularly imprinted mesoporous silica embedded with carbon dots and semiconductor quantum dots as a ratiometric fluorescent sensor for diniconazole. Biosens Bioelectron 96:121–126
Huang Y, Feng H, Liu W, Zhang S, Tang C, Chen J, Qian Z (2017) Cation-driven luminescent self-assembled dots of copper nanoclusters with aggregation-induced emission for β-galactosidase activity monitoring. J Mater Chem B 5:5120–5127
Huang Y, Liu W, Feng H et al (2016) Luminescent nanoswitch based on organic-phase copper nanoclusters for sensitive detection of trace amount of water in organic solvents. Anal Chem 88:7426–7434
Li Z, Guo S, Lu C (2015) A highly selective fluorescent probe for sulfide ions based on aggregation of Cu nanocluster induced emission enhancement. Analyst 140:2719–2725
Tang C, Feng H, Huang Y, Qian Z (2017) Reversible luminescent nanoswitches based on aggregation-induced emission enhancement of silver nanoclusters for luminescence turn-on assay of inorganic pyrophosphatase activity. Anal Chem 89(9):4994–5002
Su X, Liu J (2017) pH-guided self-assembly of copper nanoclusters with aggregation-induced emission. ACS Appl Mater Interfaces 9:3902–3910
Mohabbati-Kalejahi E, Azimirad V, Bahrami M, Ganbari A (2012) A review on creatinine measurement techniques. Talanta 97:1–8
Randviir EP, Banks CE (2013) Analytical methods for quantifying creatinine within biological media. Sensors Actuators B Chem 183:239–252
Luque de Castro MD, Priego-Capote F (2018) The analytical process to search for metabolomics biomarkers. J Pharm Biomed Anal 147:341–349
Paroni R, Fermo I, Cighetti G, Ferrero CA, Carobene A, Ceriotti F (2004) Creatinine determination in serum by capillary electrophoresis. Electrophoresis 25:463–468
Harlan R, Clarke W, Di Bussolo JM, Kozak M, Straseski J, Meany DL (2010) An automated turbulent flow liquid chromatography-isotope dilution mass spectrometry (LC-IDMS) method for quantitation of serum creatinine. Clin Chim Acta 411:1728–1734
Wen T, Zhu W, Xue C et al (2014) Novel electrochemical sensing platform based on magnetic field-induced self-assembly of Fe3O4@polyaniline nanoparticles for clinical detection of creatinine. Biosens Bioelectron 56:180–185
Rao H, Lu Z, Gel H, Liu X, Chen B et al (2017) Electrochemical creatinine sensor based on a glassy carbon electrode modified with a molecularly imprinted polymer and Ni@polyaniline nanocomposite. Microchim Acta 184:261–269
Mohammadi S, Khayatian G (2015) Highly selective and sensitive photometric creatinine assay using silver nanoparticles. Microchim Acta 182:1379–1386
Leung EMK, Chan W (2014) A novel reversed-phase HPLC method for the determination of urinary creatinine by pre-column derivatization with ethyl chloroformate: comparative studies with the standard Jaffé and isotope-dilution mass spectrometric assays. Anal Bioanal Chem 406:1807–1812
Mathew MS, Joseph K (2017) Green synthesis of gluten-stabilized fluorescent gold quantum clusters: application as turn-on sensing of human blood creatinine. ACS Sustain Chem Eng 5:4837–4845
He Y, Zhang X, Yu H (2015) Gold nanoparticles-based colorimetric and visual creatinine assay. Microchim Acta 182:2037–2043
Benkert A, Scheller F, Schössler W et al (2000) Development of a creatinine ELISA and an amperometric antibody-based creatinine sensor with a detection limit in the nanomolar range. Anal Chem 72:916–921
Wua BY, Wanga CW, Chena PC, Chang HT (2017) Glutathione assisted preparation of gold nanoclusters using minimum amount of protein. Sensors Actuators B 238:1258–1265
Wang WX, Wu Y, Li HW (2017) Regulation on the aggregation-induced emission (AIE) of DNA-templated silver nanoclusters by BSA and its hydrolysates. J Colloid Interface Sci 505:577–584
Sharma AC, Jana T, Kesavamoorthy R, Shi S, Virji M (2004) A general photonic crystal sensing motif: creatinine in bodily fluids. J Am Chem Soc 126:2971–2977
Rajamanikandan R, Ilanchelian M (2018) Protein protected red emittive copper nanoclusters as a fluorometric probe for highly sensitive biosensing of creatinine. Anal Methods 10:3666–3674
Hanif S, John P, Gao W, Saqib M, Qi L, Xu G (2016) Chemiluminescence of creatinine /H2O2/Co2+ and its application for selective creatinine detection. Biosens Bioelectron 75:347–351
Jayasekhar P, Raichur AM, Doble M (2018) Synthesis and characterization of biocompatible carbon-gold (C-au ) nanocomposites and their biomedical applications as an optical sensor for creatinine detection and cellular imaging. Sensors Actuators B 258:1267–1278
Zuo Y, Yang Y, Zhu Z, He W, Aydin Z (2011) Determination of uric acid and creatinine in human urine using hydrophilic interaction chromatography. Talanta 83:1707–1710
Talalak K, Noiphung J, Songjaroen T, Chailapakul O (2015) A facile low-cost enzymatic paper-based assay for the determination of urine creatinine. Talanta 144:915–921
Li TJ, Chen PY, Nien PC, Lin CY, Vittal R, Ling TR, Ho KC (2012) Preparation of a novel molecularly imprinted polymer by the sol-gel process for sensing creatinine. Anal Chim Acta 711:83–90
Yang C, Liu C, Cao YM, Yan XP (2014) Metal–organic framework MIL-100(Fe) for artificial kidney application. RSC Adv 6:40824–40827
Acknowledgements
Authors thank the University of Tabriz for the support provided. Roghayeh Jalili as a postdoc researcher gratefully acknowledges use of the services and facilities of the University of Tabriz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 381 kb)
Rights and permissions
About this article
Cite this article
Jalili, R., Khataee, A. Aluminum(III) triggered aggregation-induced emission of glutathione-capped copper nanoclusters as a fluorescent probe for creatinine. Microchim Acta 186, 29 (2019). https://doi.org/10.1007/s00604-018-3111-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3111-0