Abstract
Abstract
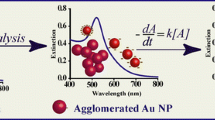
In the present study, colloidal gold nanoparticles (AuNPs) were synthesized by the citrate reduction method. The physical properties and their stability were studied in the various buffer systems at different pH and time intervals. Monodisperse AuNPs were used to assess their stability and aggregation in 0.1 M of buffers. We analyzed the stability of the colloidal gold nanoparticles in borate, phosphate, Tris–citrate and Tris–HCl buffers with variable pH and time-dependent manner. For understanding the stability and aggregation of AuNPs in different buffers, AuNPs and buffer mixture was incubated at 4 °C for different time-periods. The surface plasmon resonance (SPR) spectra and colorimetric changes were recorded at time interval. Absorbance spectra revealed that different ratios of AuNPs in borate buffer were stable at pH 7.5 to 9.3. Wine red colour with maximum absorbance at 520 nm was obtained due to lack of the aggregation. The phosphate buffer was not suitable for monodisperse gold nanoparticles and got aggregated. In Tris–citrate buffer, the absorbance A520 (*P < 0. 05 and ***P < 0. 001) and correlation coefficient changed with incubation time and λmax shifts to longer wavelengths at pH 8.5. Tris–HCl buffer revealed significant changes A520 with incubation period and did not correlate with time. There were changes in A520 with incubation time and λmax shifts towards longer wavelength at pH 4.0 while AuNPs was stable at pH 10.5. The phosphate buffer, Tris–citrate buffer and Tris–HCl buffer made complexes and got aggregated colorimetric response was shifted wine red color to blue –black color.
Graphical Abstract



















Similar content being viewed by others
Abbreviations
- AuNPs:
-
Gold nanoparticles
- TEM:
-
Transmission electron microscopy
- SPR:
-
Surface plasmon resonance
- HMD:
-
Heavy metal ions detection
- SES:
-
Surface enhanced spectroscopy
- DLS:
-
Dynamic light scattering
- UV–Vis:
-
Ultraviolet–Visible spectroscopy
- EM:
-
Electromagnetic radiation
- SPR:
-
Surface plasmon resonance
- MES:
-
4-Morpholine ethane sulphonic, acid hemi sodium salt
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- DDL:
-
Diffuse double layer
- DLVO:
-
Derjaguin–Landau–Verwey–Overbeek
- PEG-SH:
-
Poly(ethylene glycol) methyl ether thiol
References
K. Saha, S. S. Agasti, C. Kim, X. Li, and V. M. Rotello (2012). Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112(5), 2739–2779.
H. Tyagi, A. Kushwaha, A. Kumar, and M. Aslam (2016). A facile pH controlled citrate-based reduction method for gold nanoparticle synthesis at room temperature. Nanoscale Res. Lett. 11(1), 362.
R. McCaffrey, H. Long, Y. Jin, A. Sanders, W. Park, and W. Zhang (2014). Template synthesis of gold nanoparticles with an organic molecular cage. J. Am. Chem. Soc. 136(5), 1782–1785.
N. Li, P. Zhao, and D. Astruc (2014). Anisotropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angew. Chem. Int. Ed. 53(7), 1756–1789.
X. Liu, H. Xu, H. Xia, and D. Wang (2012). Rapid seeded growth of monodisperse, quasi-spherical, citrate-stabilized gold nanoparticles via H2O2 reduction. Langmuir 28(38), 13720–13726.
R. Campardelli, G. Della Porta, L. Gomez, S. Irusta, E. Reverchon, and J. Santamaria (2014). Au–PLA nanocomposites for photothermally controlled drug delivery. J. Mater. Chem. B 2(4), 409–417.
J. Lee, S. R. Ahmed, S. Oh, J. Kim, T. Suzuki, K. Parmar, S. S. Park, J. Lee, and E. Y. Park (2015). A plasmon-assisted fluoro-immunoassay using gold nanoparticle-decorated carbon nanotubes for monitoring the influenza virus. Biosens. Bioelectron. 64, 311–317.
C. Schleh, M. Semmler-Behnke, J. Lipka, A. Wenk, S. Hirn, M. Schäffler, G. Schmid, U. Simon, and W. G. Kreyling (2012). Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 6(1), 36–46.
A. M. Alkilany and C. J. Murphy (2010). Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? J. Nanopart. Res. 12(7), 2313–2333.
A. Habib, M. Tabata, and Y. G. Wu (2005). Formation of gold nanoparticles by good’s buffers. Bull. Chem. Soc. Jpn. 78(2), 262–269.
T. L. Moore, L. Rodriguez-Lorenzo, V. Hirsch, S. Balog, D. Urban, C. Jud, B. Rothen-Rutishauser, M. Lattuada, and A. Petri-Fink (2015). Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 44(17), 6287–6305.
S. A. Edwards and D. R. Williams (2004). Double layers and interparticle forces in colloid science and biology: analytic results for the effect of ionic dispersion forces. Phys. Rev. Lett. 92(24), 248303.
M. Boström, D. R. M. Williams, and B. W. Ninham (2001). Specific ion effects: why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 87(16), 168103.
M. A. Butkus and D. Grasso (1998). Impact of aqueous electrolytes on interfacial energy. J. Colloid Interface Sci. 200(1), 172–181.
J. Turkevich, P. C. Stevenson, and J. Hillier (1953). The formation of colloidal gold. J. Phys. Chem. 57(7), 670–673.
J. Liu and Y. Lu (2006). Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 1(1), 246.
M. Kalita, S. Balivada, V. P. Swarup, C. Mencio, K. Raman, U. R. Desai, D. Troyer, and B. Kuberan (2014). A nanosensor for ultrasensitive detection of oversulfated chondroitin sulfate contaminant in heparin. J. Am. Chem. Soc. 136(2), 554–557.
J. L. Yao, G. P. Pan, K. H. Xue, D. Y. Wu, B. Ren, D. M. Sun, J. Tang, X. Xu, and Z. Q. Tian (2000). A complementary study of surface-enhanced Raman scattering and metal nanorod arrays. Pure Appl. Chem. 72(1–2), 221–228.
F. Y. Alzoubi, J. Y. Alzouby, M. K. Alqadi, H. A. Alshboul, and K. M. Aljarrah (2015). Synthesis and characterization of colloidal gold nanoparticles controlled by the pH and ionic strength. Chin. J. Phys. 53(5), 100801-1.
P. J. De Temmerman, E. Van Doren, E. Verleysen, Y. Van der Stede, M. A. D. Francisco, and J. Mast (2012). Quantitative characterization of agglomerates and aggregates of pyrogenic and precipitated amorphous silica nanomaterials by transmission electron microscopy. J. Nanobiotechnol. 10(1), 24.
H. Schiffter and G. Lee (2007). Single-droplet evaporation kinetics and particle formation in an acoustic levitator. Part 1: evaporation of water microdroplets assessed using boundary-layer and acoustic levitation theories. J. Pharm. Sci. 96(9), 2274–2283.
X. Ji, X. Song, J. Li, Y. Bai, W. Yang, and X. Peng (2007). Size control of gold nanocrystals in citrate reduction: the third role of citrate. J. Am. Chem. Soc. 129(45), 13939–13948.
C. Li, D. Li, G. Wan, J. Xu, and W. Hou (2011). Facile synthesis of concentrated gold nanoparticles with low size-distribution in water: temperature and pH controls. Nanoscale Res. Lett. 6(1), 440.
J. Huang, W. Liu, D. S. Dolzhnikov, L. Protesescu, M. V. Kovalenko, B. Koo, S. Chattopadhyay, E. V. Shenchenko, and D. V. Talapin (2014). Surface functionalization of semiconductor and oxide nanocrystals with small inorganic oxoanions (PO43–, MoO42–) and polyoxometalate ligands. ACS Nano 8(9), 9388–9402.
K. Rahme, M. T. Nolan, T. Doody, G. P. McGlacken, M. A. Morris, C. O’Driscoll, and J. D. Holmes (2013). Highly stable PEGylated gold nanoparticles in water: applications in biology and catalysis. RSC Adv. 3(43), 21016–21024.
Acknowledgements
The authors would like to thank the Director, ICAR-National Dairy Research Institute, Karnal-132001(Haryana). India and gratefully acknowledged. The authors thank for providing the facilities for the research.
Author information
Authors and Affiliations
Contributions
SS conducted all experiments and prepared the draft of manuscript. RS supervised the work and revised the manuscript, provided general guidance and advice. All authors contributed to discussing and commenting on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no any conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sangwan, S., Seth, R. Synthesis, Characterization and Stability of Gold Nanoparticles (AuNPs) in Different Buffer Systems. J Clust Sci 33, 749–764 (2022). https://doi.org/10.1007/s10876-020-01956-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01956-8