Abstract
Non-native tree species are often used as ornamentals in urban landscapes. However, their root-associated fungal communities remain yet to be examined in detail. Here, we compared richness, diversity and community composition of ectomycorrhizosphere fungi in general and ectomycorrhizal (EcM) fungi in particular between a non-native Pinus nigra and a native Quercus macrocarpa across a growing season in urban parks using 454-pyrosequencing. Our data show that, while the ectomycorrhizosphere community richness and diversity did not differ between the two host, the EcM communities associated with the native host were often more species rich and included more exclusive members than those of the non-native hosts. In contrast, the ectomycorrhizosphere communities of the two hosts were compositionally clearly distinct in nonmetric multidimensional ordination analyses, whereas the EcM communities were only marginally so. Taken together, our data suggest EcM communities with broad host compatibilities and with a limited numbers of taxa with preference to the non-native host. Furthermore, many common fungi in the non-native Pinus were not EcM taxa, suggesting that the fungal communities of the non-native host may be enriched in non-mycorrhizal fungi at the cost of the EcM taxa. Finally, while our colonization estimates did not suggest a shortage in EcM inoculum for either host in urban parks, the differences in the fungi associated with the two hosts emphasize the importance of using native hosts in urban environments as a tool to conserve endemic fungal diversity and richness in man-made systems.





Similar content being viewed by others
References
Amend AS, Seifert KA, Samson R, Bruns TD (2010) Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proceedings of the National Academy of Sciences of the United States of America 107:13748–13753
Angel S, Sheppard SC, Civco DL (2005) The dynamics of global urban expansion. The World Bank, Washington, DC
Appleton B, Koci J, French S, Lestyan M, Harris R (2003) Mycorrhizal fungal inoculation of established street trees. J Arboric 29:107–110
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Arnolds E (1991) Decline of ectomycorrhizal fungi in Europe. Agric Ecosyst Environ 35:209–244
Ash C, Jasny BR, Roberts L, Stone R, Sugden A (2008) Reimagining cities—introduction. Science 319:739–739
Bahram M, Köljalg U, Kohout P, Mirshahvaladi S, Tedersoo L (2013) Ectomycorrhizal fungi of exotic pine plantations in relation to native host trees in Iran: evidence of host range expansion by local symbionts to distantly related host taxa. Mycorrhiza 23:11–19
Baxter JW, Dighton J (2001) Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host-symbiont culture conditions. New Phytol 152:139–159
Baxter JW, Dighton J (2005) Diversity–functioning relationships in ectomycorrhizal fungal communities. In: Dighton J, White JF, Oudemans P (eds) The fungal community—its role and organization in the ecosystem, 3rd edn. CRC Press, Boca Raton, pp 383–398
Baxter JW, Pickett STA, Carreiro MM, Dighton J (1999) Ectomycorrhizal diversity and community structure in oak forest stands exposed to contrasting anthropogenic impacts. Can J Bot 77:771–782
Bainard LD, Klironomos JN, Gordon AM (2011) The mycorrhizal status and colonization of 26 tree species growing in urban and rural environments. Mycorrhiza 21:92–96
Berry D, Mahfoudh KB, Wagner M, Loy A (2011) Barcoded primers used in multiplex amplicon pyrosequencing bias amplification. Appl Environ Microbiol 77:7846–7849
Boerner REJ, DeMars BG, Leicht PN (1996) Spatial patterns of mycorrhizal infectiveness of soils long a successional chronosequence. Mycorrhiza 6:79–90
Bonfante P, Balestrini R, Martino E, Perotto S, Plassard C, Mousain D (1998) Morphological analysis of early contacts between pine roots and two ectomycorrhizal Suillus strains. Mycorrhiza 8:1–10
Byrd KB, Parker VT, Vogler DR, Cullings KW (2000) The influence of clear-cutting on ectomycorrhizal fungus diversity in a lodgepole pine (Pinus contorta) stand, Yellowstone National Park, Wyoming, and Gallatin National Forest, Montana. Can J Bot 78:149–156
Chou-Chou M, Grace LJ (1983) Characterization and identification of mycorrhizas of Douglas-fir in New Zealand. Eur J For Pathol 13:251–260
Cousins JR, Hope D, Gries C, Stutz JC (2003) Preliminary assessment of arbuscular mycorrhizal fungal diversity and community structure in an urban ecosystem. Mycorrhiza 13:319–326
Cullings KW, Vogler DR, Parker VT, Finley SK (2000) Ectomycorrhizal specificity in a mixed Pinus contorta and Picea engelmannii forest in Yellowstone National park. Appl Environ Microbiol 66:4988–4991
Dickie IA, Bolstridge N, Cooper JA, Peltzer DA (2010) Co-invasion by Pinus and its mycorrhizal fungi. New Phytol 187:475–484
Dighton J, Tuininga AR, Gray DM, Huskins RE, Belton T (2004) Impacts of atmospheric deposition on New Jersey pine barren forest soils and communities of ectomycorrhizae. For Ecol Manag 201:133–144
Druebert C, Lang C, Valtanen K, Polle A (2009) Beech carbon productivity as driver of ectomycorrhizal abundance and diversity. Plant Cell Environ 32:992–1003
Edman M, Gustafsson M, Stenlid J, Ericson L (2004) Abundance and viability of fungal spores along a forestry gradient—responses to habitat loss and isolation? Oikos 104:35–42
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Ferrini F, Nicese FP (2002) Response of English oak (Quercus robur L.) trees to biostimulants application in the urban environment. J Arboric 28:70–75
Fierer N, Jackson JA, Vilgalys R, Jackson RB (2005) Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol 71:4117–4120
Fini A, Frangi P, Amoroso G, Piatti R, Faoro M, Bellasio C, Ferrini F (2011) Effect of controlled inoculation with specific mycorrhizal fungi from the urban environment on growth and physiology of containerized shade tree species growing under different water regimes. Mycorrhiza 21:703–719
Gebhardt S, Neubert K, Wöllecke J, Münzenburger B, Hütl RF (2007) Ectomycorrhiza communities of red oak (Quercus rubra L.) of different age in the Lusatian lignite mining district, East Germany. Mycorrhiza 17:279–290
Gehring CA, Whitham TG (1992) Reduced mycorrhizae on Juniperus monosperma with mistletoe: the influence of environmental stress and tree gender on a plant parasite and a plant–fungal mutualism. Oecologia 89:298–303
Gihring TM, Green SJ, Schadt CW (2012) Massively parallel rRNA gene sequencing exacerbates the potential for biased community diversity comparisons due to variable library sizes. Environ Microbiol 14:285–290
Gilman EF (2001) Effect of nursery production method, irrigation, and inoculation with mycorrhizae-forming fungi on establishment of Quercus virginiana. J Arboric 27:30–39
Högberg MN, Bååth E, Nordgren A, Arnebrant K, Högberg P (2003) Contrasting effects of nitrogen availability on plant carbon supply to mycorrhizal fungi and saprotrophs—a hypothesis based on field observations in boreal forest. New Phytol 160:225–238
Jacobson KM, Miller OK (1992) Physiological variation between tree associated populations of Suillus granulatus as determined by in vitro mycorrhizal synthesis experiments. Can J Bot 70:26–31
Jones MD, Durall DM, Cairney JWG (2003) Ectomycorrhizal fungal communities in young forest stands regenerating after clearcut logging. New Phytol 157:399–422
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184:438–448
Jumpponen A, Jones KL (2010) Seasonally dynamic fungal communities in Quercus macrocarpa phyllosphere differ among urban and rural environments. New Phytol 186:496–513
Jumpponen A, Jones KL, Mattox JD, Yeage C (2010a) Massively parallel 454-sequencing of fungal communities in Quercus spp. ectomycorrhizas indicates seasonal dynamics in urban and rural sites. Mol Ecol 19(Suppl. 1): 41–53
Jumpponen A, Keating K, Gadbury GL, Jones KL, Mattox JD (2010b) Multi-element fingerprinting and high throughput sequencing identify multiple elements that affect fungal communities in Quercus macrocarpa foliage. Plant Signal Behav 5:1157–1161
Karpati AS, Handel SN, Dighton J, Horton TR (2011) Quercus rubra-associated ectomycorrhizal fungal communities of disturbed urban sites and mature forests. Mycorrhiza 21:537–547
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518
Kohout P, Sýkorová Z, Bahram M, Hadincová V, Albrechtová J, Tedersoo L, Vohník M (2011) Ericaceous dwarf shrubs affect ectomycorrhizal fungal community of the invasive Pinus strobus and native Pinus sylvestris in a pot experiment. Mycorrhiza 21:403–412
Kaye JP, Groffman PM, Grimm NB, Baker LA, Pouyat RV (2006) A distinct urban biogeochemistry? Tree 21:192–199
Kranabetter JM, Hayden S, Wright EF (1999) A comparison of ectomycorrhiza communities from three conifer species planted on forest gap edges. Can J Bot 77:1193–1198
Lazarevic J, Keca N, Martinovic A (2012) Mycorrhization of containerized Pinus nigra seedlings with Suillus granulatus under open field conditions. For Syst 21:498–507
Lehto T, Zwiazek JJ (2011) Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21:71–91
Liu K-L, Kuske CR, Porras-Alfaro A, Eichorst S, Xie G (2012) Accurate, rapid taxonomic classification of fungal large subunit rRNA genes. Appl Environ Microbiol 78:1523–1533
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Marschner H, Dell B (1994) Nutrient-uptake in mycorrhizal symbiosis. Plant Soil 159:89–102
Martin-Pinto P, Pajares J, Diez J (2006) In vitro effects of four ectomycorrhizal fungi, Boletus edulis, Rhizopogon roseolus, Laccaria laccata and Lactarius deliciosus on Fusarium damping off in Pinus nigra seedlings. New For 32:323–334
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning. An integrative plant–fungal process. Chapman and Hall, New York, pp 357–423
Morris MH, Perez-Perez MA, Smith ME, Bledsoe CS (2009) Influence of host species on ectomycorrhizal communities associated with two co-occurring oaks (Quercus spp.) in a tropical cloud forest. FEMS Microbiol Ecol 69:274–287
Newbound M, Mccarthy MA, Lebel T (2010) Fungi and the urban environment: a review. Landsc Urban Plan 96:138–145
Nicholson FB, Korman MG (1997) Death from Amanita poisoning. Aust NZ J Med 27:448–449
O’Hanlon R, Harrington TJ (2012) Similar taxonomic richness but different communities in native forests and non-native plantation forests. Mycorrhiza 22:371–382
O’Hanlon R, Harrington TJ, Berch SM, Outerbridge RA (2013) Comparisons of macrofungi in plantations of Sitka spruce (Picea sitchensis) in its native range (British Columbia, Canada) versus non-native range (Ireland and Britain) show similar richness but different species composition. Can J For Res 43:450–458
Ochimaru T, Fukuda K (2007) Changes in fungal communities in evergreen broad-leaved forests across a gradient of urban to rural areas in Japan. Can J For Res 37:247–258
Pavao-Zuckerman MA (2008) The nature of urban soils and their role in ecological restoration in cities. Restor Ecol 16:642–649
Peay KG, Bruns TD, Kennedy PG, Bergemann SE, Garbelotto M (2007) A strong species–area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi. Ecol Lett 10:470–480
Pena R, Offermann C, Simon J, Naumann PS, Geßler A, Holst J, Mayer H, Kögel-Knabner I, Rennenberg H, Polle A (2010) Girdling affects ectomycorrhizal diversity and reveals functional differences of EM community composition in a mature beech forest (Fagus sylvatica). Appl Environ Microbiol 76:1831–1841
Peter M, Ayer F, Egli S (2001) Nitrogen addition in a Norway spruce stand altered macromycete sporocarp production and below-ground ectomycorrhizal species composition. New Phytol 149:311–325
Pfaffl M (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Polanco MC, Zwiazek JJ, Voicu MC (2008) Responses of ectomycorrhizal American elm (Ulmus americana) seedlings to salinity and soil compaction. Plant Soil 308:189–200
Pouyat RV, McDonnell MJ (1991) Heavy metal accumulations in forest soils along an urban–rural gradient in southeastern New York, USA. Water Air Soil Pollut 57–8:797–807
Quince C, Lanzén A, Curtis TP, Davenport RJ, Hall N, Head IM, Read LF, Sloan WT (2009) Accurate determination of microbial diversity from 454 pyrosequencing data. Nat Methods 6:639
Rao B, Marx DH, Jeffers B (2006) Response of oaks and elm to soil inoculations with mycorrhizal fungi and rhizobacteria in a nursery. Arboric Urban For 32:62–66
Read DJ (1991) Mycorrhizas in ecosystems. Experentia 47:376–390
Richard F, Millot S, Gardes M, Selosse MA (2005) Diversity and specificity of ectomycorrhizal fungi retrieved from an old-growth Mediterranean forest dominated by Quercus ilex. New Phytol 166:1011–1023
Schaefer VH (2011) Remembering our roots: a possible connection between loss of ecological memory, alien invasions and ecological restoration. Urban Ecosyst 14:35–44
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 6:e27310
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesneiwski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Van Thallinger GC, Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Simard SW, Durall DM (2004) Mycorrhizal networks: a review of their extent, function, and importance. Can J Bot 82:1140–1165
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Elsevier, Amsterdam
Stabler LB, Martin CA, Stutz JC (2001) Effect of urban expansion on arbuscular mycorrhizal fungal mediation of landscape tree growth. J Arboric 27:193–202
Tedersoo L, Suvi T, Beaver K, Kõljalg U (2007) Ectomycorrhizal fungi of the Seychelles: diversity patterns and host shifts from the native Vateriopsis seychellarum (Dipterocarpaceae) and Intsia bijuga (Caesalpiniaceae) to the introduced Eucalyptus robusta (Myrtaceae), but not Pinus caribea (Pinaceae). New Phytol 175:321–333
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Köljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490
Tedersoo L, Nilsson RH, Abarenkov K, Jairus T, Saar I, Bahram M, Bechem E, Chuyong G, Kõljalg U (2010) 454 pyrosequencing and Sanger sequencing of tropical mycorrhizal fungi provide similar results but reveal substantial methodological biases. New Phytol 188:291–301
Timonen S, Kauppinen P (2008) Mycorrhizal colonisation patterns of Tilia trees in street, nursery and forest habitats in southern Finland. Urban For Urban Green 7:265–276
Trocha L, Kalucha I, Stasinska M, Nowak W, Dabert M, Leski T, Rudawska M, Oleksyn J (2012) Ectomycorrhizal fungal communities of native and non-native Pinus and Quercus species in a common garden of 35-year-old trees. Mycorrhiza 22:121–134
Trudell SA, Edmonds RL (2004) Macrofungus communities correlate with moisture and nitrogen abundance in two old-growth conifer forests, Olympic National Park, Washington, USA. Can J Bot 82:781–800
Vellinga EC, Wolfe BE, Pringle A (2009) Global patterns of ectomycorrhizal introductions. New Phytol 181:960–973
Walker JF, Miller OK, Horton JL (2005) Hyperdiversity of ectomycorrhizal fungus assemblages on oak seedlings in mixed forests in the southern Appalachian Mountains. Mol Ecol 14:829–838
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve Bayesian Classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Watling R (2005) Fungal conservation: some impressions—a personal view. In: Dighton J, White JF, Oudemans P (eds) The fungal community—its organisation and role in the ecosystem, 3rd edn. CRC Press, Boca Raton
Whipps JM (2004) Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can J Bot 82:1198–1227
Acknowledgments
We are grateful to the city of Manhattan for permitted sampling within the city limits and Kansas State University for allowing sampling on campus. Kale Lothamer was supported by Undergraduate Research Mentoring (URM) in Ecological Genomics program (NSF Grant # 1041199). Shawn Brown was supported by GAANN (Graduate Awards in Areas of National Need) from Department of Education and GK-12 (NSF DGE-0841414, PI Ferguson) awards. This is Kansas Agricultural Experimental Station publication number 14-162-J.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table S1
Primer and dna-tag sequences used for sample specific PCR and 454-sequencing. (TXT 1 kb)
Supplemental Table S2
Putative assignments to ecological roles of the 378 genera detected in the 36 ectomycorrhosphere samples. AM – Arbuscular Mycorrhiza; ECM – EctoMycorrhizal; ENT – ENTomopahtogen; PAR – PARastitic, PAT – PAThogenic; SAP – SAProbic; UNK – UNKnown. (TXT 6 kb)
Supplemental Table S3
Taxon assignments of 188 OTUs that occurred more than once in the entire dataset. K_Sup, P_Sup, C_Sup, O_Sup, F_Sup, G_Sup refer to Ribosomal Database Project’s naïve Bayesian classifier bootstrap support for Kingdom, Phylum, Class, Order, Family and Genus, respectively. Ecology refers to assignments using list in Supplemental Table S2. Last two columns list total counts of sequences assigned to each OTU for Quercus macrocarpa and Pinus nigra. (TXT 21 kb)
Supplemental Table S4
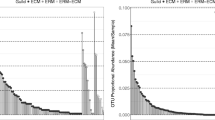
Mean (± 1 standard deviation) root and ectomycorrhizal tip densities, EcM colonization, qPCR derived inoculum load (ITS1 copy number), Good’s coverage, observed richness (SObs), Simpson’s diversity complement (1-D), Shannon’s diversity, extrapolated richness estimate (Chao I), and evenness as estimated by Simpson’s equitability (ED) for all ectomycorrhizosphere inhabiting fungi. Note that only the root and ectomycorrhizal tip densities differ in the second sampling in August, other comparisons of the two hosts at each sampling time (one way ANOVA) are non-significant (see Figs 1-2). Compare these ectomycorrhizosphere analyses to those for ectomycorrhizal fungi only (Supplemental Table S5). (TXT 1 kb)
Supplemental Table S5
Mean (± 1 standard deviation) Good’s coverage, observed richness (SObs), Simpson’s diversity complement (1-D), Shannon’s diversity, extrapolated richness estimate (Chao I), and evenness as estimated by Simpson’s equitability (ED) for all ectomycorrhizal fungi. Note that the two hosts did not differ in repeated measures ANOVA. In contrast, many richness and diversity estimators differed in early sampling in June and marginally so in the late sampling in October (see Fig. 3). (TXT 0 kb)
Supplemental Table S6
Frequencies (mean ± standard deviation) of the 43 most abundant OTUs analyzed for OTU-level responses in June, August, and October 2011. (TXT 5 kb)
Rights and permissions
About this article
Cite this article
Lothamer, K., Brown, S.P., Mattox, J.D. et al. Comparison of root-associated communities of native and non-native ectomycorrhizal hosts in an urban landscape. Mycorrhiza 24, 267–280 (2014). https://doi.org/10.1007/s00572-013-0539-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-013-0539-2