Abstract
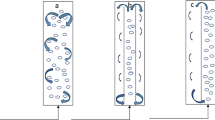
The unicellular green microalgae, Haematococcus pluvialis, has been examined as a microbial source for the production of astaxanthin, which has been suggested as a food supplement for humans and is also prescribed as an ingredient in eye drops because of its powerful anti-oxidant properties. In this study, we estimated the effects of the slope of a V-shaped bottom design, the volumetric flow rate of air, height/diameter (H/D) ratio, and diameter of an air sparger on the performance of a photo-bioreactor. These parameters were selected because they are recognized as important factors effecting the mixing that produces increased cell density in the reactor. The mixing effect can be measured by changes in optical density in the bioreactor over a period of time. A 6 L indoor photo-bioreactor was prepared in a short time period of 24 h for the performance study. A bioreactor designed with a V-shaped bottom with a slope of 60° showed an optical density change of 0.052 at 680 nm, which was sixfold less than the change in a photo-bioreactor designed with a flat bottom. Studies exploring the effects of bioreactor configuration and a porous metal sparger with a 10 μm pore size showed the best performance at an H/D ratio of 6:1 and a sparger diameter of 1.3 cm, respectively. The optimal rate of air flow was 0.2 vvm. The indoor culture of microalgae in the photo-bioreactor was subsequently carried for an application study using the optimal values established for the important factors. The indoor culture system was composed of a light source controlled according to cell phase, a carbon dioxide feeder, a bag-type reactor with an H/D ratio of 6:1, and a temperature controller. Results demonstrated the efficient production of microalgal cells and astaxanthin in the amounts of 2.62 g/L and 78.37 mg/L, respectively, when using adequate hydrodynamic mixing. Furthermore, the optimal design of a photo-bioreactor can be applied for the phototropic culturing of other microalgae for mass production.







Similar content being viewed by others
References
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin; applications for human health and nutrition. Trends Biotechnol 21:210–216
Kobayashi M, Sakamoto Y (1999) Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett 21:265–269
Margalith PZ (1999) Production of ketocarotenoids by microalgae. Appl Microbiol Biotechnol 51:431–438
Olaizola M (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol Eng 20:459–466
Kakizono T, Kobayashi M, Nagai S (1992) Effect of carbon/nitrogen ratio on encystment accompanied with astaxanthin formation in a green alga, Haematococcus pluvialis. J Ferment Bioeng 74:403–405
Fábregas J, Domínguez A, Maseda A, Otero A (2003) Interactions between irradiance and nutrient availability during astaxanthin accumulation and degradation in Haematococcus pluvialis. Appl Microbiol Biotechnol 61:545–551
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoids biosynthesis by oxidative stress in acetate induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microb 59:867–873
Kamonpan K, Artiwan S, Sorawit P, Prasert P (2007) Photoautotrophic high-density cultivation of vegetative cells of Haematococcus pluvialis in airlift bioreactor. Bioresource Technol 98:288–295
Kerati I, Sorawit P, Prasert P (2009) Flat panel airlift photo-bioreactors for cultivation of vegetative cells of microalga Haematococcus pluvialis. J Biotechnol 142:227–232
Kang CD, Han SJ, Choi SP, Sim SJ (2010) Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioproc Biosyst Eng 33:133–139
Asterio SM, Antonio CG, Francisco GC, Emilio MG, Yusuf C (1999) Comparative evaluation of compact photo-bioreactors for large-scale monoculture of microalgae. J Biotechnol 70:249–270
Molina E, Acie′n FG, Garcı′a F, Chisti Y (1999) Photo-bioreactors: light regime, mass transfer, and scaleup. J Biotechnol 70:231–247
Molina E, Ferna′ndez J, Acie′n FG, Chisti Y (2001) Tubular photo-bioreactor design for algal cultures. J Biotechnol 92:113–131
Haque MW, Nigam KDP, Joshi JB (1986) Optimum gas sparger design for bubble columns with a low height-to-diameter ratio. Chem Eng J 33:63–69
José ADC, Mercedes GG, Miguel GG (2007) Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl Microbiol Biotechnol 74:1163–1174
Miguel O (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000 liter outdoor photo-bioreactors. J Appl Phycol 12:499–506
Doucha J, Lívanský K (2009) Outdoor open thin-layer microalgalphoto-bioreactor: potential productivity. J Appl Phycol 21:111–117
Cerón MC, García-Malea MC, Rivas J, Acien FG, Fernandez JM, Del RE, Guerrero MG, Molina E (2007) Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl Microbiol Biotechnol 74:1112–1119
Sarada R, Vidhyavathi R, Usha D, Ravishankar GA (2006) An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J Agric Food Chem 54:7585–7588
Ping H, James D, James B (2007) Astaxanthin Accumulation in the green alga Haematococcus pluvialis: effects of cultivation parameters. J Integr Plant Biol 49:447–451
Kang CD, Lee JS, Park TH, Sim SJ (2005) Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl Microbiol Biotechnol 68:237–241
Lee J, Lee SY, Park S, Middelberg APJ (1999) Control of fed-batch fermentations. Biotechnol Adv 17:29–48
Yuan JP, Chen F (2000) Purification of trans-astaxanthin from a high-yielding astaxanthin ester-producing strain of the microalga Haematococcuspluvialis. Food Chem 68:443–448
Humberto JS, Johnpirt S (1984) Carbon dioxide inhibition of photosynthetic growth of Chlorella. J Gen Microbiol 130:2833–2838
Mario G, John B, John AR (2005) CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Plant Biol 56:99–131
Doran PM (1995) Bioprocess engineering principles. Academic press, London
Lee HS, Seo MW, Kim ZH, Lee CG (2006) Determining the best specific light uptake rates for the lumostatic cultures in bubble column photo-bioreactors. Enzyme Microb Tech 39:447–452
Kang CD, Lee JS, Park TH, Sim SJ (2007) Complementary limiting factors of astaxanthin synthesis during photoautotrophic induction of Haematococcuspluvialis: C/N ratio and light intensity. Appl Microbiol Biotechnol 74:987–994
Lee HS, Kim ZH, Jung SE, Kim JD, Lee CG (2006) Specific light uptake rate can be served as a scale up parameter in photo-bioreactor operations. J Microbiol Biotechnol 16:1890–1896
Acknowledgments
This work was financially supported by the grant (DG2-201) from the Carbon Dioxide Reduction & Sequestration Research Center, one of the 21st Century Frontier Programs, and the Advanced Biomass R&D Center (ABC) of Korea Grant (2010-0029728), funded by the Ministry of Science and Technology of the Korean government of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, J.J., Choi, S.P., Kim, B.W. et al. Optimal design of scalable photo-bioreactor for phototropic culturing of Haematococcus pluvialis . Bioprocess Biosyst Eng 35, 309–315 (2012). https://doi.org/10.1007/s00449-011-0616-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-011-0616-x