Abstract
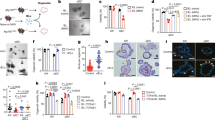
Intraepithelial lymphocytes (IELs) have been considered to play a key role in the defense system of the small intestine. Its mechanism has not been made sufficiently clear. Studies on IELs have been extremely limited to functions of αβ T-cell receptor (αβTCR) IELs (αβ-IELs). Since, in the mouse duodenum and jejunum, γδ-IELs consist 75 % of IELs, it thus would be inappropriate to argue the mechanism without extensive discussions over the functions of γδ-IELs. In previous studies, we found that the anti-CD3 monoclonal antibody (mAb) injection induced DNA fragmentation in intestinal epithelial cells (IECs) and DNA repair immediately after, that these responses were reproduced by anti-γδTCR mAb not by anti-αβTCR mAb and that the DNA fragmentation was induced by Granzyme B secreted by IELs, totally independent of Perforin. To further explore the functions of IELs in situ, we undertook experiments exclusively focused on IELs, on their changes and ultimate fate after the stimulation in mouse in vivo system. The current study demonstrated that the injected anti-CD3 mAb bound to CD3 on IELs, that the mAb activated γδ-IELs, leading to their degranulation, that changes occurred irreversibly in IELs and finally that activated IELs died in situ. γδ-IELs could be considered to respond to various stimulations most likely without the need of accessory cells (“always ready for rapid response”), to die in situ (“disposable”) and thus to respond to the stimulation only once (“a one-shot responder”). These characteristics of γδ-IELs are important to further elucidate the functions of γδ-IELs in the intestinal defense system.









Similar content being viewed by others
References
Alcover A, Alarcon B (2000) Internalization and intracellular fate of TCR-CD3 complexes. Crit Rev Immunol 20:325–346
Andrew DP, Rott LS, Kilshaw PJ, Butcher EC (1996) Distribution of alpha 4 beta 7 and alpha E beta 7 integrins on thymocytes, intestinal epithelial lymphocytes and peripheral lymphocytes. Eur J Immunol 26:897–905
Beagley KW, Husband AJ (1998) Intraepithelial lymphocytes: origins, distribution, and function. Crit Rev Immunol 18:237–254
Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, Sharmanov AT, Yamamoto M, McGhee JR, Elson CO et al (1995) Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol 154:5611–5619
Boismenu R, Havran WL (1994) Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science 266:1253–1255
Boyer C, Auphan N, Luton F, Malburet JM, Barad M, Bizozzero JP, Reggio H, Schmitt-Verhulst AM (1991) T cell receptor/CD3 complex internalization following activation of a cytolytic T cell clone: evidence for a protein kinase C-independent staurosporine-sensitive step. Eur J Immunol 21:1623–1634
Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB (1994) Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature 372:190–193
Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R (2002) Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA 99:14338–14343
Cheroutre H, Lambolez F, Mucida D (2011) The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol 11:445–456
Davies MD, Parrott DM (1981) Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut 22:481–488
Gramzinski RA, Adams E, Gross JA, Goodman TG, Allison JP, Lefrancois L (1993) T cell receptor-triggered activation of intraepithelial lymphocytes in vitro. Int Immunol 5:145–153
Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P (1991a) Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med 173:471–481
Guy-Grand D, Malassis-Seris M, Briottet C, Vassalli P (1991b) Cytotoxic differentiation of mouse gut thymodependent and independent intraepithelial T lymphocytes is induced locally. Correlation between functional assays, presence of perforin and granzyme transcripts, and cytoplasmic granules. J Exp Med 173:1549–1552
Haas W, Pereira P, Tonegawa S (1993) Gamma/delta cells. Annu Rev Immunol 11:637–685
Hiroi T, Fujihashi K, McGhee JR, Kiyono H (1995) Polarized Th2 cytokine expression by both mucosal gamma delta and alpha beta T cells. Eur J Immunol 25:2743–2751
Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, Aukerman SL, Devine PL, Whitehead RH, Pierce GF (1994) Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest 94:1764–1777
Ishikawa H, Li Y, Abeliovich A, Yamamoto S, Kaufmann SH, Tonegawa S (1993) Cytotoxic and interferon gamma-producing activities of gamma delta T cells in the mouse intestinal epithelium are strain dependent. Proc Natl Acad Sci USA 90:8204–8208
Ismail AS, Behrendt CL, Hooper LV (2009) Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol 182:3047–3054
Janeway CA Jr, Jones B, Hayday A (1988) Specificity and function of T cells bearing gamma delta receptors. Immunol Today 9:73–76
Lefrancois L (1991a) Extrathymic differentiation of intraepithelial lymphocytes: generation of a separate and unequal T-cell repertoire? Immunol Today 12:436–438
Lefrancois L (1991b) Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol 147:1746–1751
Lefrancois L, Puddington L (1995) Extrathymic intestinal T-cell development: virtual reality? Immunol Today 16:16–21
Lefrancois L, Barrett TA, Havran WL, Puddington L (1994) Developmental expression of the alpha IEL beta 7 integrin on T cell receptor gamma delta and T cell receptor alpha beta T cells. Eur J Immunol 24:635–640
Legendre V, Guimezanes A, Buferne M, Barad M, Schmitt-Verhulst AM, Boyer C (1999) Antigen-induced TCR-CD3 down-modulation does not require CD3delta or CD3gamma cytoplasmic domains, necessary in response to anti-CD3 antibody. Int Immunol 11:1731–1738
Luton F, Buferne M, Schmitt-Verhulst AM, Boyer C (1994) Developmental control of T-cell receptor internalization. Thymus 23:15–25
Luton F, Legendre V, Gorvel JP, Schmitt-Verhulst AM, Boyer C (1997) Tyrosine and serine protein kinase activities associated with ligand-induced internalized TCR/CD3 complexes. J Immunol 158:3140–3147
Ogata M, Oomori T, Soga H, Ota Y, Itoh A, Matsutani T, Nanno M, Suzuki R, Itoh T (2009) DNA repair after DNA fragmentation in mouse small intestinal epithelial cells. Cell Tissue Res 335:371–382
Ogata M, Ota Y, Matsutani T, Nanno M, Suzuki R, Itoh T (2013) Granzyme B-dependent and perforin-independent DNA fragmentation in intestinal epithelial cells induced by anti-CD3 mAb-activated intra-epithelial lymphocytes. Cell Tissue Res 352:287–300
Pabst R (1987) The anatomical basis for the immune function of the gut. Anat Embryol (Berl) 176:135–144
Penney L, Kilshaw PJ, MacDonald TT (1995) Regional variation in the proliferative rate and lifespan of alpha beta TCR + and gamma delta TCR + intraepithelial lymphocytes in the murine small intestine. Immunology 86:212–218
Poussier P, Julius M (1994) Intestinal intraepithelial lymphocytes: the plot thickens. J Exp Med 180:1185–1189
Reinis M, Morra M, Funaro A, Di Primio R, Malavasi F (1997) Functional associations of CD38 with CD3 on the T-cell membrane. J Biol Regul Homeost Agents 11:137–142
Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC (1996) T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci USA 93:11774–11779
Rubin B, Llobera R, Gouaillard C, Alcover A, Arnaud J (2000) Dissection of the role of CD3gamma chains in profound but reversible T-cell receptor down-regulation. Scand J Immunol 52:173–183
Shi L, Kraut RP, Aebersold R, Greenberg AH (1992) A natural killer cell granule protein that induces DNA fragmentation and apoptosis. J Exp Med 175:553–566
Simecka JW (1998) Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev 34:235–259
Taguchi T, Aicher WK, Fujihashi K, Yamamoto M, McGhee JR, Bluestone JA, Kiyono H (1991) Novel function for intestinal intraepithelial lymphocytes. Murine CD3+, gamma/delta TCR + T cells produce IFN-gamma and IL-5. J Immunol 147:3736–3744
Tamura A, Soga H, Yaguchi K, Yamagishi M, Toyota T, Sato J, Oka Y, Itoh T (2003) Distribution of two types of lymphocytes (intraepithelial and lamina-propria-associated) in the murine small intestine. Cell Tissue Res 313:47–53
Yaguchi K, Kayaba S, Soga H, Yamagishi M, Tamura A, Kasahara S, Ohara S, Satoh J, Oka Y, Toyota T, Itoh T (2004) DNA fragmentation and detachment of enterocytes induced by anti-CD3 mAb-activated intraepithelial lymphocytes. Cell Tissue Res 315:71–84
Acknowledgements
This work was in part supported by a Grant-in-aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (20590181 to M.O. and 21590207 to T.I.) and the Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogata, M., Ota, Y., Nanno, M. et al. Activation of intra-epithelial lymphocytes; their morphology, marker expression and ultimate fate. Cell Tissue Res 356, 217–230 (2014). https://doi.org/10.1007/s00441-013-1786-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1786-4