Abstract
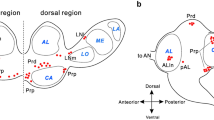
Circadian locomotor activity rhythms of the cockroach Leucophaea maderae are driven by two bilaterally paired and mutually coupled pacemakers that reside in the optic lobes of the brain. Transplantation studies have shown that this circadian pacemaker is located in the accessory medulla (AMe), a small neuropil of the medulla of the optic lobe. The AMe is densely innervated by about 12 anterior pigment-dispersing-hormone-immunoreactive (PDH-ir) medulla (PDHMe) neurons. PDH-ir neurons are circadian pacemaker candidates in the fruitfly and cockroach. A subpopulation of these neurons also appears to connect both optic lobes and may constitute at least one of the circadian coupling pathways. To determine whether PDHMe neurons directly connect both accessory medullae, we injected rhodamine-labeled dextran as neuronal tracer into one AMe and performed PDH immunocytochemistry. Double-labeled fibers in the anterior, shell, and internodular neuropil of the AMe contralaterally to the injection site showed that PDH-ir fibers directly connect both accessory medullae. This connection is formed by three anterior PDHMe neurons of each optic lobe, which, thus, fulfill morphological criteria for a direct circadian coupling pathway. Our double-label studies also showed that all except one of the midbrain projection areas of anterior PDHMe neurons were innervated ipsilaterally and contralaterally. Thus, anterior PDHMe neurons seem to play multiple roles in generating circadian rhythms. They also deliver timing information output and perform mutual pacemaker coupling in L. maderae.









Similar content being viewed by others
References
Chiba Y, Tomioka K (1987) Insect circadian activity with special reference to the localization of the pacemaker. Zool Sci 4:945–954
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riem JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean glands: identification by an antiserum against a synthetic PDH. Cell Tissue Res 250:377–387
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci USA 92:612–616
Helfrich-Förster C (1998) Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioral study of disconnected mutants. J Comp Physiol [A] 182:435–453
Helfrich-Förster C, Stengl M, Homberg U (1998) Organization of the circadian system in insects. Chronobiol Int 15:567–594
Homberg U, Würden S, Dircksen H, Rao KR (1991) Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Homberg U, Reischig T, Stengl M (2003) Neural organization of the circadian system of the cockroach Leucophaea maderae. Chronobiol Int 20:577–591
Loesel R, Homberg U (1998) Sustained oscillations in an insect visual system. Naturwissenschaften 85:238–240
Loesel R, Homberg U (2001) Anatomy and physiology of neurons with processes in the accessory medulla of the cockroach Leucophaea maderae. J Comp Neurol 439:193–207
Lupien M, Marshall S, Leser W, Pollack GS, Honegger HW (2003) Antibodies against the PER protein of Drosophila label neurons in the optic lobe, central brain, and thoracic ganglia of the crickets Teleogryllus commodus and Teleogryllus oceanicus. Cell Tissue Res 312:377–391
Nishiitsutsuji-Uwo J, Pittendrigh CS (1968) Central nervous system control of circadian rhythmicity in the cockroach. II. The optic lobes, locus of the driving oscillator? Z Vergl Physiol 58:14–46
Page TL (1978) Interactions between bilaterally paired components of the cockroach circadian system. J Comp Physiol [A] 124:225–236
Page TL (1982) Transplantation of the cockroach circadian pacemaker. Science 216:73–75
Page TL (1983a) Effects of optic-tract regeneration on internal coupling in the circadian system of the cockroach. J Comp Physiol [A] 153:353–363
Page TL (1983b) Regeneration of the optic tracts and circadian pacemaker activity in the cockroach Leucophaea maderae. J Comp Physiol [A] 152:231–240
Page TL (1984) Neuronal organization of a circadian clock in the cockroach Leucophaea maderae. Photoperiodic regulation of insect and molluscan hormones (Ciba foundation symposium 104). Pitman, London, pp 115–135
Page TL, Caldarola PC, Pittendrigh CS (1977) Mutual entrainment of bilaterally distributed circadian pacemakers. Proc Natl Acad Sci USA 74:1277–1281
Park JH, Helfrich-Förster C, Lee G, Liu L, Rosbash M, Hall JC (2000) Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci USA 97:3608–3613
Petri B, Stengl M (1997) Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci 17:4087–4093
Petri B, Stengl M (2001) Phase response curves of a molecular model oscillator: implications for mutual coupling of paired oscillators. J Biol Rhythms 16:125–141
Petri B, Stengl M, Würden S, Homberg U (1995) Immunocytochemical characterization of the accessory medulla in the cockroach Leucophaea maderae. Cell Tissue Res 282:3–19
Petri B, Homberg U, Loesel R, Stengl M (2002) Evidence for a role of GABA and Mas-allatotropin in photic entrainment of the circadian clock of the cockroach Leucophaea maderae. J Exp Biol 205:1459–1469
Reischig T, Stengl M (1996) Morphology and pigment-dispersing hormone immunocytochemistry of the accessory medulla, the presumptive circadian pacemaker of the cockroach Leucophaea maderae: a light- and electron-microscopic study. Cell Tissue Res 285:305–319
Reischig T, Stengl M (2002) Optic lobe commissures in a three-dimensional brain model of the cockroach Leucophaea maderae: a search for the circadian coupling pathways. J Comp Neurol 443:388–400
Reischig T, Stengl M (2003a) Ectopic transplantation of the accessory medulla restores circadian locomotor rhythms in arrhythmic cockroaches (Leucophaea maderae). J Exp Biol 206:1877–1886
Reischig T, Stengl M (2003b) Ultrastructure of pigment-dispersing hormone-immunoractive neurons in a three-dimensional model of the accessory medulla of the cockroach (Leucophaea maderae). Cell Tissue Res 314:421–435
Roberts SK (1974) Circadian rhythms in cockroaches: effects of optic lobe lesions. J Comp Physiol [A] 88:21–30
Rodriguez J, Deinhard F (1960) Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology 12:316–317
Roth RL, Sokolove PG (1975) Histological evidence for direct connections between the optic lobes of the cockroach Leucophaea maderae. Brain Res 87:23–39
Sokolove PG (1975) Localization of the cockroach optic lobe circadian pacemaker with microlesions. Brain Res 87:13–21
Stanewsky R (2002) Clock mechanisms in Drosophila. Cell Tissue Res 309:11–26
Stengl M (1995) Pigment-dispersing hormone-immunoreactive fibers persist in crickets which remain rhythmic after bilateral transection of the optic stalks. J Comp Physiol [A] 176:217–228
Stengl M, Homberg U (1994) Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leucophaea maderae share properties with circadian pacemaker neurons. J Comp Physiol [A] 175:203–213
Ushirogawa H, Abe Y, Tomioka K (1997) Circadian locomotor rhythms in the cricket, Gryllodes sigillatus. II. Interactions between bilaterally paired circadian pacemakers. Zoolog Sci 14:729–736
Wiedenmann G, Loher W (1984) Circadian control of singing in crickets: two different pacemakers for early-evening and before-dawn activity. J Insect Physiol 30:145–151
Acknowledgements
We are grateful to Heinrich Dircksen (University of Stockholm, Sweden) for providing the anti-β-PDH antiserum.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) grants STE 531/7-1, 2, 3, and Human Science Frontier
Rights and permissions
About this article
Cite this article
Reischig, T., Petri, B. & Stengl, M. Pigment-dispersing hormone (PDH)-immunoreactive neurons form a direct coupling pathway between the bilaterally symmetric circadian pacemakers of the cockroach Leucophaea maderae. Cell Tissue Res 318, 553–564 (2004). https://doi.org/10.1007/s00441-004-0927-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-0927-1