Abstract
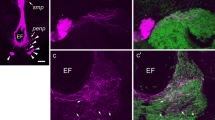
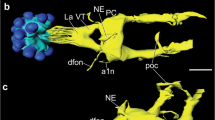
We describe labeling of neurons in the central nervous system of two cricket species, Teleogryllus commodus and T. oceanicus, with both mono- and polyclonal antibodies against the PER protein. Western blots reveal that the monoclonal antibodies recognize a single protein with a molecular weight of approximately 94 kDa, i.e., similar to that of the PER protein of the moth, Anterea pernii. Neurons and their processes are labeled both in the optic lobes and in the central brain. Processes occur in the accessory medulla, the medulla, and proximal lamina, in the central complex, in the non-glomerular neuropil, and in the retrocerebral complex, suggesting that PER-containing neurons form a widely distributed network. Neurons and processes were also labeled in the meso- and metathoracic ganglia. Four to six PER-immunoreactive (ir) neurons with processes in the accessory medulla were double labeled by an antibody against pigment-dispersion factor (PDF), a peptide that is implicated in circadian rhythmicity in Drosophila. In the central brain, projections of fibers labeled by the anti-PER and anti-PDF antibodies were mainly distinct, with overlap only in a few restricted regions. In most neurons, including those projecting into the accessory medulla, PER labeling was restricted to the cytoplasm and there was no indication of circadian variation in the intensity of staining.









Similar content being viewed by others
References
Abe Y, Ushirogawa H, Tomioka K (1997) Circadian locomotor rhythms in the cricket, Gryllodes sigillatus. I. Localization of the pacemaker and the photoreceptor. Zool Sci 14:719–727
Alt S, Ringo J, Talyn B, Bray W, Dowse H (1998) The period gene controls courtship song cycles in Drosophila melanogaster. Anim Behav 56:87–97
Bae K, Jin X, Maywood ES, Hastings MH Reppert SM, Weaver DR (2001) Differential functions of mPerl, mPer2, and mPer3 in the SCN circadian clock. Neuron 30:525–536
Balakrishnan R, Pollack GS (1996) Recognition of courtship song in the field cricket, Teleogryllus oceanicus. Anim Behav 51:353–366
Bartos M, Allgäuer C, Eckert M, Honegger HW (1994) The antennal motor system of crickets: proctolin in slow and fast motoneurons as revealed by double labeling. Eur J Neurosci 6:825–836
Bausenwein B, Müller RM, Heisenberg H (1994) Behaviour-dependent activity labeling in the central complex of Drosophila melanogaster during controlled visual stimuli. J Comp Neurol 340:255–268
Chiba Y, Tomioka K (1987) Insect circadian activity with special reference to the localization of the pacemaker. Zool Sci 4:945–954
Colot HV, Hall JC, Rosbash M (1988) Interspecific comparison of the period gene of Drosophilia reveals large blocks of non-conserved coding DNA. EMBO J 7:3929–3937
Colwell CS, Page TL (1990) A circadian rhythm in neuronal activity can be recorded from the central nervous system of the cockroach. J Comp Physiol A 166:643–649
Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96:271–290
Ewer J, Frisch B, Hamblen-Coyle MJ, Rosbash M, Hall JC (1992) Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cell's influence on circadian behavioral rhythms. J Neurosci 12:3321–3349
Frisch B, Fleissner G, Fleissner G, Brandes C, Hall JC (1996) Staining in the brain of Pachymorph sexguttata mediated by an antibody against a Drosophila clock-gene product: labeling of cells with possible importance for the beetle's circadian rhythms. Cell Tissue Res 286:411–429
Giebultowicz JM (2000) Molecular mechanism and cellular distribution of insect circadian clocks. Annu Rev Entomol 45:769–793
Hedwig B (2000) Control of cricket stridulation by a command neuron: efficacy depends on behavioral state. J Neurophysiol 83:712–722
Heisenberg M (1994) Central brain functions in insects: genetic studies on the mushroom bodies and central complex in Drosophila. In: Schilberger K, Elsner N (eds) Neural basis of behavioural adaptations. Fischer, Stuttgart, pp 61–79
Helfrich-Förster C (1995) The period clock gene is expressed in central nervous system neurons which also produce a neuropeptide that reveals the projections of circadian pacemaker cells within the brain of Drosophila melanogaster. Proc Natl Acad Sci U S A 92:12–16
Helfrich-Förster C (1998) Robust circadian rhythmicity of Drosophila melanogaster requires the presence of lateral neurons: a brain-behavioural study of disconnected mutants. J Comp Physiol A 182:435–453
Helfrich-Förster C, Homberg U (1993) Pigment-dispersing hormone-immunoreactive neurons in the nervous system of wild-type Drosophila melanogaster and of several mutants with altered circadian rhythmicity. J Comp Neurol 337:177–190
Helfrich-Förster C, Stengl M, Homberg U (1998) Organization of the circadian system in insects. Chronobiol Int 15:567–594
Hennig RM (1990) Neuronal control of the forewing in two different behaviours: stridulation and flight in the cricket, Teleogryllus commodus. J Comp Physiol A 167:617–627
Hennig RM, Otto D (1995/96) Distributed control of song pattern generation in crickets revealed by lesion to the thoracic ganglia. Zool (Jena) 99:268–276
Homberg U (1985) Interneurons of the central complex in the bee brain (Apis mellifica L.). J Insect Physiol 31:251–264
Homberg U, Müller M (1995) Neurons in the central complex in the locust brain are sensitive to polarized light. In: Burrows M, Matheson T, Newland PC, Schuppe H (eds) Nervous systems and behaviour. Thieme, Stuttgart, p 279
Homberg U, Würden S, Dircksen H, Rao KR (1991) Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res 266:343–357
Honegger HW, Schürmann FW (1975) Cobalt sulphide staining of optic fibers in the brain of the cricket, Gryllus campestris. Cell Tissue Res 159:213–225
Honegger HW, Leser W, Loher W, Siwicki KK (1991) Labeling of cells in the CNS of the cricket Teleogryllus commodus by an antibody to Drosophila per-protein. Soc Neurosci Abstr 17:1239
Honegger HW, Leser W, Schiffelholz T (1995) Neurons labeled with an antiserum against the period-gene product and control of circadian calling in Teleogryllus commodus. In: Elsner N, Menzel R (eds) Proc 23rd Neurobiol Conf Goettingen, vol II. Thieme, Stuttgart, p 601
Ilius M, Wolf R, Heisenberg M (1994) The central complex of Drosophila melanogaster is involved in flight control: studies on mutants and mosaics of the gene ellipsoid open. J Neurogenet 9:189–206
Kaneko M, Hall JC (2000) Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422:66–94
Konopka RJ, Benzer S (1971) Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A 68:2112–2116
Konopka RJ, Kyriacou CP, Hall JC (1996) Mosaic analysis in the Drosophila CNS of circadian and courtship-song rhythms affected by a period clock mutation. J Neurogenet 11:17–139
Kostron B, Kaltenhauser U, Seibel B, Bräunig P, Honegger HW (1996) Localization of bursicon in CCAP-immunoreactive cells in the thoracic ganglia of the cricket Gryllus bimaculatus. J Exp Biol 199:367–377
Krishnan B, Dryer SE, Hardin PE (1999) Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400:375–378
Kyriacou CP, Hall JC (1980) Circadian rhythm mutations in Drosophila melanogaster affect short-term fluctuations in the male's courtship song. Proc Natl Acad Sci U S A 77:6729–6733
Loher W (1972) Circadian control of stridulation in the cricket Teleogryllus commodus Walker. J Comp Physiol 79:173–190
Lupien M, Pollack GS (1998) Correlation between circadian and ultradian rhythms in crickets: role for the period gene? Proc Fifth Intl Congr Neuroethol, p 262
Nishiitsutsuji-Uwo J, Pittendrigh CS (1968) Central nervous system control of circadian rhythmicity in the cockroach. III. The optic lobes, locus of driving oscillation? Z Vergl Physiol 58:14–46
Page TL (1982) Transplantation of the cockroach circadian pacemaker. Science 216:73–75
Page TL (1983) Regeneration of the optic tracts and pacemaker activity in the cockroach Leucophaea maderae. J Comp Physiol 152:231–240
Panda S, Hogenesch JB, Kay SA (2002) Circadian rhythms from flies to human. Nature 417:329–335
Petri B, Stengl M (1997) Pigment-dispersing hormone shifts the phase of the circadian pacemaker of the cockroach Leucophaea maderae. J Neurosci 17:4087–4093
Petri B, Stengl M, Würden S, Homberg U (1995) Immunocytochemical characterization of the accessory medulla in the cockroach Leucophaea maderae. Cell Tissue Res 282:3–19
Plautz JD, Kaneko M, Hall JC, Kay SF (1997) Independent photoreceptive circadian clocks throughout Drosophila. Science 278:1632–1735
Rao KR, Mohrherr CJ, Riehm JP, Zanow CA, Norton S, Johnson L, Tarr GE (1987). Primary structure of an analog of crustacean pigment-dispersing hormone from the lubber grasshopper Romalea microptera. J Biol Chem 262:2672–2675
Rence B, Loher W (1975) Arhythmically singing crickets: thermoperiodic reentrainment after bilobectomy. Science 190:385–387
Reppert SM, Tsai T, Roca AL, Sauman I (1994) Cloning of a structural and functional homolog of the circadian clock gene period from the giant silkmoth Antheraea pernyi. Neuron 13:1167–1176
Sauman I, Reppert SM (1996) Circadian clock neurons in the silkmoth Anrheraea pernyi: Novel mechanisms of period protein regulation. Neuron 17:889–900
Schürmann FW, Ottersen OP, Honegger HW (2000) Glutamate-like immunoreactivity marks compartments of the mushroom bodies in the brain of the cricket. J Comp Neurol 418:227–239
Siwicki KK, Eastman C, Petersen G, Rosbash M, Hall JC (1988) Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic change in the visual system. Neuron 1:141–150
Siwicki KK, Schwartz WJ, Hall JC (1992) An antibody to the Drosophila period protein labels antigens in the suprachiasmatic nucleus of the rat. J Neurogenet 8:33–42
Sokolove PG (1975) Locomotory and stridulatory circadian rhythms in the cricket Teleogryllus commodus. J Insect Physiol 21:537–558
Sokolove PG, Loher W (1975) Role of eyes, optic lobes, and pars intercerebralis in locomotory and stridulatory circadian rhythms of Teleogryllus commodus. J Insect Physiol 21:785–799
Stengl M (1995) Pigment-dispersing hormone-immunoreactive fibers persist in crickets which remain rhythmic after bilateral transection of the optic stalks. J Comp Physiol A 176:217–228
Stengl M, Homberg U (1994) Pigment-dispersing hormone-immunoreactive neurons in the cockroach Leukophaea maderae share properties with circadian pacemaker neurons. J Comp Physiol A 175:203–213
Strauss RU, Heisenberg M (1993) A higher control center of locomotor behavior in the Drosophila brain. J Neurosci 13:1852–1861
Thoma DP, Bloch G, Moore D, Robinson GE (2000) Changes in the period mRNA levels in the brain and division of labor in honey bee colonies. Proc Natl Acad Sci U S A 97:6914–6919
Tomioka K, Chiba Y (1992) Characterization of an optic lobe circadian pacemaker by in situ and in vitro recording of neural activity in the cricket, Gryllus bimaculatus. J Comp Physiol A 171:1–7
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354
Vitzthum H, Homberg U (1998) Immunocytochemical demonstration of locust tachykinin-related peptides in the central complex of the locust brain. J Comp Neurol 390:455–469
Wiedenmann G (1983) Splitting in a circadian activity rhythm: the expression of bilaterally paired oscillators. J Comp Physiol 150:51–60
Wiedenmann G (1984) Circadian control of singing in crickets: two different pacemakers for early-evening and before-dawn activity. J Insect Physiol 30:145–151
Williams JLD (1975) Anatomical studies of the insect nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool Lond 76:67–86
Wise S, Davis NT, Tyndale E, Noveral J, Folwell MG, Bedian V, Emery IF, Siwicki KK (2002) Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J Comp Neurol 447:366–380
Würden S, Homberg U (1995) Immunochemical mapping of serotonin and neuropeptides in the accessory medulla of the locust Schistocerca gregaria. J Comp Neurol 362:305–319
Zerr DM, Hall JC, Rosbash M, Siwicki KK (1990) Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci 10:2749–2762
Zheng B, Albrecht U, Kaasik K, Sage M, Lu W, Vaishnav S, Li Q, Sun ZS, Eichele G, Bradley A, Lee CC (2001) Nonredundant roles of the mPer1 and mPer 2 genes in the mammalian circadian clock. Cell 105:683–694
Acknowledgements
We are grateful to Dr. K.K. Siwicki for the generous gift of the anti-PER antibodies, to Dr. Rao for the anti-PDF antiserum, and to Drs. K.K. Siwicki and F.-W. Schürmann for critically reading the manuscript. We appreciate the excellent work of K.A. Klukas in producing the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mathieu Lupien and Stephanie Marshall appear in alphabetical order. They contributed equally to the project
This work was supported by a grant of the Deutsche Forschungsgemeinschaft (Ho463/20–1) and from the NSF (grant number IBN 0080084 001) to H.W.H., and from the Fonds pour la Formation de Chercheurs et l'Aide à la Recherche of Québec to G.S.P. and S. Hekimi.
Rights and permissions
About this article
Cite this article
Lupien, M., Marshall, S., Leser, W. et al. Antibodies against the PER protein of Drosophila label neurons in the optic lobe, central brain, and thoracic ganglia of the crickets Teleogryllus commodus and Teleogryllus oceanicus . Cell Tissue Res 312, 377–391 (2003). https://doi.org/10.1007/s00441-003-0720-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-003-0720-6