Abstract
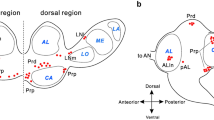
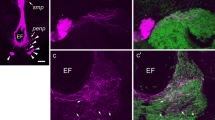
Adult diapause in the bean bug, Riptortus pedestris, is controlled by the photoperiod, which is received by retinal cells in the central region of the compound eyes. To resolve the afferent neural pathways involved in the photoperiodic response, we examine fibre projections from the photoperiodic receptors to the brain and investigate the roles of the posterior optic tract (POT) in the photoperiodic response. Reduced-silver impregnation and synapsin immunolabelling revealed that the medulla was divided into nine strata: the outer layer comprises 4 strata, the inner layer comprises 4 strata and a serpentine layer separates the inner and outer layers. Biotin injection revealed that retinal fibres from the central region of the compound eye terminated in either the central part of the lamina or the central part of the medulla 3rd or 4th layer. Biotin injection into the central part of the medulla labelled 5 distinct afferent pathways: two terminated in a region of ipsilateral anterior protocerebrum, while the other three had contralateral projections. One pathway ran through the POT and connected to the bilateral medulla serpentine layers. When the POT was surgically severed, diapause incidence under short-day conditions was significantly reduced compared to that observed following a sham operation. However, an incision at a posterior part of the medulla and lobula boundary, as a control experiment, did not affect the photoperiodic response. These results suggest that photoperiodic signals from the central region of the compound eye are transferred to neurons with fibres running in the POT for photoperiodic response in R. pedestris.










Similar content being viewed by others
Abbreviations
- AL:
-
Antennal lobe
- AOT:
-
Anterior optic tract
- AOTU:
-
Anterior optic tubercle
- CA:
-
Calyces of the mushroom body
- CC:
-
Central complex
- CE:
-
Compound eye
- DAT:
-
Dorsal anterior tract
- DT:
-
Dorsal tract
- LA:
-
Lamina
- LO:
-
Lobula
- MB:
-
Mushroom body
- ME:
-
Medulla
- OL:
-
Optic lobe
- PDF:
-
Pigment-dispersing factor
- PLF:
-
Posterior lateral fibres
- POT:
-
Posterior optic tract
- SEZ:
-
Subesophageal zone
References
El Jundi B, Huetteroth W, Kurylas AE, Schachtner J (2009) Anisometric brain dimorphism revisited: implementation of a volumetric 3D standard brain in Manduca sexta. J Comp Neurol 517:210–225
Fischbach KF, Dittrich APM (1989) The optic lobe of Drosophila melanogaster. I. A Golgi analysis of wild-type structure. Cell Tissue Res 258:441–475
Fischbach KF, Lyly-Hünerberg (1983) I. Genetic dissection of the anterior optic tract of Drosophila melanogaster. Cell Tissue Res 231:551–563
Goto SG, Shiga S, Numata H (2010) Photoperiodism in insects: perception of light and the role of clock genes. In: Photoperiodism: The biological calendar. Oxford University Press, Oxford, pp 258–286
Hamanaka Y, Yasuyama K, Numata H, Shiga S (2005) Synaptic connections between pigment-dispersing factor-immunoreactive neurons and neurons in the pars lateralis of the blow fly Protophormia terraenovae. J Comp Neurol 491:390–399
Hamanaka Y, Kinoshita M, Homberg U, Arikawa K (2012) Immunocytochemical localization of amines and GABA in the optic lobe of the butterfly, Papilio xuthus. PLoS ONE 7(7):e41109
Harris WA, Stark WS, Walker JA (1976) Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol 256:415–439
Hertel H, Maronde U (1987) Processing of visual information in the honeybee brain. In: Neurobiology and Behavior of Honeybees. Springer, Berlin, pp 141–157
Homberg U, Hofer S, PfeiVer K, Gebhardt S (2003) Organization and neural connections of the anterior optic tubercle in the brain of the locust, Schistocerca gregaria. J Comp Neurol 462:415–430
Honegger HW, Schürmann FW (1975) Cobalt sulphide staining of optic fibres in the brain of the cricket, Gryllus campestris. Cell Tissue Res 159:213–225
Horridge GA (1968) A note on the number of retinula cells of Notonecta. J Comp Phys A 61:259–262
Ikeno T, Tanaka SI, Numata H, Goto SG (2010) Photoperiodic diapause under the control of circadian clock genes in an insect. BMC Biol 8:116
Ikeno T, Katagiri C, Numata H, Goto SG (2011a) Causal involvement of mammalian-type cryptochrome in the circadian cuticle deposition rhythm in the bean bug Riptortus pedestris. Insect Mol Biol 20:409–415
Ikeno T, Numata H, Goto SG (2011b) Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem Biophys Res Commun 410:394–397
Ikeno T, Numata H, Goto SG (2011c) Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J Insect Physiol 57:935–938
Ikeno T, Ishikawa K, Numata H, Goto SG (2013) Circadian clock gene, Clock, is involved in the photoperiodic response of the bean bug Riptortus pedestris. Physiol Entomol 38:157–162
Ikeno T, Numata H, Goto SG, Shiga S (2014) Involvement of the brain region containing pigment-dispersing factor-immunoreactive neurons in the photoperiodic response of the bean bug Riptortus pedestris. J Exp Biol 217:453–462
Jander U, Jander R (2002) Allometry and resolution of bee eyes (Apoidea). Arthropod Struct Dev 30:179–193
Kamano S (1991) Riptortus clavatus (Thunberg) (Bean bug). In: Yushima T, Kamano S, Tamaki Y, editors. Rearing methods of insects. Tokyo: Japan Plant Protection Association, pp 46–49 (in Japanese)
Koshitaka H, Kinoshita M, Vorobyev M, Arikawa K (2008) Tetrachromacy in a butterfly that has eight varieties of spectral receptors. J Biol Sci 275:947–954
Koštál V, Zavodska R, Denlinger D (2009) Clock genes period and timeless are rhythmically expressed in brains of newly hatched, photosensitive larvae of the fly, Sarcophaga crassipalpis. J Insect Physiol 55:408–414
Meinertzhagen IA, Hanson TE (1993) The development of the optic lobe. In: The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, New York, pp 1363–1491
Mobbs PG (1982) The brain of the honeybee Apis mellifera. I. The connections and spatial organization of the mushroom bodies. Philos Trans R Soc Lond 298:309–354
Morante J, Desplan C (2004) Building a projection map for photoreceptor neurons in the Drosophila optic lobes. Semin Cell Dev Biol 15:137–143
Morita A, Numata H (1997) Distribution of photoperiodic receptors in the compound eyes of the bean bug, Riptortus clavatus. J Comp Phys A 180:181–185
Numata H, Hidaka T (1982) Photoperiodic control of adult diapause in the bean bug, Riptortus clavatus Thunberg (Heteroptera: Coreidae). I. Reversible induction and termination of diapause. J Appl Entomol Zool 17:530–538
Numata H, Shiga S, Morita A (1997) Photoperiodic receptors in Arthropods. Zool Sci 14:187–197
Otsuka N (1962) Histologisch-entwicklungsgeschichtliche Untersuchungen an Mauthnerschen Zellen von Fischen. Z Zellforch Microsk Anat Histochem 58:33–50
Paulk AC, Phillips-Portillo J, Dacks AM, Fellous JM, Gronenberg W (2008) The processing of color, motion, and stimulus timing are anatomically segregated in the bumblebee brain. J Neruosci 28:6319–6332
Paulk AC, Dacks AM, Gronenberg W (2009) Color processing in the medulla of the bumblebee (Apidae: Bombus impatiens). J Comp Neurol 513:441–456
Pfeiffer K, Kinoshita M (2012) Segregation of visual inputs from different regions of the compound eye in two parallel pathways through the anterior optic tubercle of the bumblebee (Bombus ignitus). J Comp Neurol 520:212–229
Reischig T, Stengl M (2002) Optic lobe commissures in a three-dimensional brain model of the cockroach Leucophaea maderae: a search for the circadian coupling pathways. J Comp Neurol 443:388–400
Reischig T, Stengl M (2003) Ectopic transplantation of the accessory medulla restores circadian locomotor rhythms in arrhythmic cockroaches (Leucophaea maderae). J Exp Biol 206:1877–1886
Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802
Ribi WA (1987) Anatomical identification of spectral receptor types in the retina and lamina of the Australian orchard butterfly, Papilio aegeus. Cell Tissue Res 247:393–407
Ribi WA, Scheel M (1981) The second and third optic ganglia of the worker bee: Golgi studies of the neuronal elements in the medulla and lobula. Cell Tissue Res 221:17–43
Roth RL, Sokolove PG (1975) Histological evidence for direct connections between the optic lobes of the cockroach Leucophaea maderae. Brain Res 87:23–39
Rybak J, Menzel R (1993) Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the alpha-lobe. J Comp Neurol 334:444–465
Sakamoto T, Uryu O, Tomioka K (2009) The clock gene period plays an essential role in photoperiodic control of nymphal development in the cricket Modicogryllus siamensis. J Biol Rhythms 24:379–390
Salcedo E, Huber A, Henrich S, Chadwell LV, Chou WH, Paulsen R, Britt SG (1999) Blue-and green-absorbing visual pigments of Drosophila: Ectopic expression and physiological characterization of the R8 photoreceptor cell-specific Rh5 and Rh6 rhodopsins. J Neurosci 19:10716–10726
Saunders DS (1984) Photoperiodic time measurement in Sarcophaga argyrostoma: An attempt to use daily temperature cycles to distinguish external from internal coincidence. J Comp Physiol A 154:789–794
Saunders DS (2012) Insect photoperiodism: seeing the light. J Physiol Entomol 37:207–218
Shiga S, Numata H (2007) Neuroanatomical approaches to the study of insect photoperiodism. Photochem Photobiol 83:76–86
Shiga S, Numata H (2009) Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae. J Exp Biol 212:867–877
Shimokawa K, Numata H, Shiga S (2008) Neurons important for the photoperiodic control of diapause in the bean bug, Riptortus pedestris. J Comp Physiol A 194:751–762
Stark W S (1975) Spectral selectivity of visual response alterations mediated by interconversions of native and intermediate photopigments in Drosophila. J Comp Physiol 96:343–356
Stark WS, Walker JA, Harris WA (1976) Genetic dissection of the photoreceptor system in the compound eye of Drosophila melanogaster. J Physiol 256:415
Stehlík J, Závodská R, Shimada K, Šauman I, Koštál V (2008) Photoperiodic induction of diapause requires regulated transcription of timeless in the larval brain of Chymomyza costata. J Biol Rhythms 23:129–139
Strausfeld NJ (1970) Golgi studies on insects. Part II: The optic lobes of Diptera. Philos Trans R Soc Lond B 258:135–223
Strausfeld NJ (1971) The organization of the insect visual system (Light microscopy). Z Zellforsch Mikrosk Anat 121:377–441
Strausfeld NJ (1976) Mosaic organization, layers and visual pathways in the insect brain. In: Neural principles in vision. Springer, Berlin, pp 245–279
Strausfeld NJ, Okamura J (2007) Visual system of calliphorid flies: organization of optic glomeruli and their lobula complex efferents. J Comp Neurol 500:166–188
Talarico F, Romeo M, Massolo A, Brandmayr P, Zetto T (2007) Morphometry and eye morphology in three species of Carabus (Coleoptera: Carabidae) in relation to habitat demands. J Zool Syst Evol Res 45:33–38
Tamaki S, Takemoto S, Uryu O, Kamae Y, Tomioka K (2013) Opsins are involved in nymphal photoperiodic responses in the cricket Modicogryllus siamensis. J Physiol Entomol 38:163–172
Wada S (1974) Spezielle randzonale ommatidien von Calliphora Erythrocephala meig. (diptera calliphoridae): Architektur der zentralen rhabdomeren-kolumne und topographie im komplexauge. Int J Insect Morphol Embryol 3:397–424
Yamaguchi S, Claude D, Martin H (2010) Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc Natl Acad Sci U S A 107:5634–5639
Yukizane M, Tomioka K (1995) Neural pathways involved in mutual interactions between optic lobe circadian pacemakers in the cricket Gryllus bimaculatus. J Comp Physiol 176:601–610
Yukizane M, Kaneko A, Tomioka K (2002) Electrophysiological and morphological characterization of the medulla bilateral neurons that connect bilateral optic lobes in the cricket, Gryllus bimaculatus. J Insect Physiol 48:631–641
Acknowledgements
We thank Dr. Michiyo Kinoshita from Sokendai for technical advice on biotin staining. This work was supported by a Grant-in Aid for Scientific Research (B), 26292175 by the Japan Society for Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xi, J., Toyoda, I. & Shiga, S. Afferent neural pathways from the photoperiodic receptor in the bean bug, Riptortus pedestris . Cell Tissue Res 368, 469–485 (2017). https://doi.org/10.1007/s00441-016-2565-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-016-2565-9