Abstract
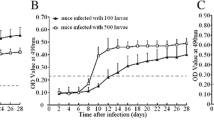
The excretory-secretory (ES) antigens from Trichinella spiralis muscle larvae (ML) are the most commonly used diagnostic antigens for trichinellosis, but anti-Trichinella IgG antibodies cannot be detected until 2–3 weeks after infection; there is an obvious window period between Trichinella infection and antibody positivity. Intestinal infective larvae (IIL) are the first invasive stage during Trichinella infection, and their ES antigens are firstly exposed to the immune system and might be the early diagnostic markers of trichinellosis. The aim of this study was to evaluate the early diagnostic values of IIL ES antigens for trichinellosis. The IIL were collected from intestines of infected mice at 6 h postinfection (hpi), and IIL ES antigens were prepared by incubation for 18 h. Anti-Trichinella IgG antibodies in mice infected with 100 ML were detectable by ELISA with IIL ES antigens as soon as 10 days postinfection (dpi), but ELISA with ML ES antigens did not permit detection of infected mice before 12 dpi. When the sera of patients with trichinellosis at 19 dpi were assayed, the sensitivity (100 %) of ELISA with IIL ES antigens was evidently higher than 75 % of ELISA with ML ES antigens (P < 0.05) The specificity (96.86 %) of ELISA with IIL ES antigens was also higher than 89.31 % of ELISA with ML ES antigens (P < 0.05). The IIL ES antigens provided a new source of diagnostic antigens and could be considered as a potential early diagnostic antigen for trichinellosis.


Similar content being viewed by others
References
Appleton JA, Bell RG, Homan W, Van Knapen F (1991) Consensus on Trichinella spiralis antigens and antibodies. Parasitol Today 7(8):190–192
Aronstein WS, Lewis SA, Norden AP, Dalton JP, Strand M (1986) Molecular identity of a major antigen of Schistosoma mansoni which cross-reacts with Trichinella spiralis and Fasciola hepatica. Parasitology 92(1):133–151
Bell RG (1998) The generation and expression of immunity to Trichinella spiralis in laboratory rodents. Adv Parasitol 41:149–217
Bioreau P, Vayssier M, Fabien JF, Perret C, Calamel M, Soule C (1997) Characterization of eleven antigenic groups in Trichinella genus and identification of stage and species markers. Parasitology 115(6):641–651
Bolas-Fernandez F, Dea-Ayuela MA, Connolly B, Robinson MW (2009) Micro-environmental conditions modulate protein secretion and infectivity of the Trichinella spiralis L1 larva. Vet Parasitol 159(3–4):236–239
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruschi F, Tassi C, Pozio E (1990) Parasite-specific antibody response in Trichinella sp. 3 human infection: a one year follow-up. Am J Trop Med Hyg 43(2):186–193
Campbell W (1983) Trichinella and trichinosis. Plenum Press, New York, p 75
Capron A, Biguet J, Vernes A, Afchain D (1968) Structure antigénique des helminthes: aspects immunologiques des relations hoste-parasite. Pathol Biol 16:121–138
Chan SW, Ko RC (1988) Comparison between standard ELISA and dot-ELISA for serodiagnosis of human trichinosis. Trans R Soc Trop Med Hyg 82(6):892–894
Cui J, Wang ZQ, Zhang D (2003) Study on specific diagnostic antigens in excretory-secretory products from muscle larvae of Trichinella spiralis. Chin J Parasitol Parasit Dis 21(5):268–271
Cui J, Li N, Wang ZQ, Jiang P, Lin XM (2011) Serodiagnosis of experimental sparganum infections of mice and human sparganosis by ELISA using ES antigensof Spirometra mansoni spargana. Parasitol Res 108:1551–1556
Cui J, Liu RD, Wang L, Zhang X, Jiang P, Liu MY, Wang ZQ (2013) Proteomic analysis of surface proteins of Trichinella spiralis muscle larvae by two-dimensional gel electrophoresis and mass spectrometry. Parasit Vectors 6:355
Cui J, Wei T, Liu LN, Zhang X, Xin Q, Zhang ZF, Wang ZQ (2014) Molecular characterization of a Spirometra mansoni antigenic polypeptide gene encoding a 28.7 kDa protein. Parasitol Res 113(9):3511–3516
Cui J, Wang L, Sun GG, Liu LN, Zhang SB, Liu RD, Xi Z, Jiang P, Wang ZQ (2015) Characterization of a Trichinella spiralis 31 kDa protein and its potential application for the serodiagnosis of trichinellosis. Acta Trop 142:57–63
Dea-Ayuela MA, Romaris F, Ubeira FM, Rama-Iniguez S, Martinez-Fernandez AR, Bolas F (2001) Possible presence of common tyvelose-containing glycans in Trichinella L1 larvae and embryonated eggs of several nematodes. Parasite 8:S120–S122
Dupouy-Camet J, Kociecka W, Bruschi F, Bolas-Fernandez F, Pozio E (2002) Opinion on the diagnosis and treatment of human trichinellosis. Expert Opin Pharmacother 3(8):1117–1130
Gamble HR, Bessonov AS, Cuperlovic K, Gajadhar AA, van Knapen F, Noeckler K, Schenone H, Zhu X (2000) International Commission on Trichinellosis: recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol 93(3–4):393–408
Gamble HR, Pozio E, Bruschi F, Nockler K, Kapel CM, Gajadhar AA (2004) International Commission on Trichinellosis: recommendations on the use of serological tests for the detection of Trichinella infection in animals and man. Parasite 11(1):3–13
Gomez-Morales MA, Ludovisi A, Amati M, Cherchi S, Pezzotti P, Pozio E (2008) Validation of an enzyme-linked immunosorbent assay for diagnosis of human trichinellosis. Clin Vaccine Immunol 15(11):1723–1729
Kapel CM, Gamble HR (2000) Infectivity, persistence, and antibody response to domestic and sylvatic Trichinella spp. in experimentally infected pigs. Int J Parasitol 30(2):215–221
Linder E, Thors C, Lundin L, Ljungstrom I, Farah S, Hagi H, Dias F (1992) Schistosome antigen gp50 is responsible for serological cross-reactivity with Trichinella spiralis. J Parasitol 78(6):999–1005
Liu LN, Jing FJ, Cui J, Fu GY, Wang ZQ (2013a) Detection of circulating antigen in serum of mice infected with Trichinella spiralis by an IgY-IgM mAb sandwich ELISA. Exp Parasitol 133(2):150–155
Liu RD, Wang ZQ, Wang L, Long SR, Ren HJ, Cui J (2013b) Analysis of differentially expressed genes of Trichinella spiralis larvae activated by bile and cultured with intestinal epithelial cells using real-time PCR. Parasitol Res 112(12):4113–4120
Liu RD, Cui J, Liu XL, Jiang P, Sun GG, Zhang X, Long SR, Wang L, Wang ZQ (2015) Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Trop 150:79–86
Moller LN, Petersen E, Gamble HR, Kapel CM (2005) Comparison of two antigens for demonstration of Trichinella spp. antibodies in blood and muscle fluid of foxes, pigs and wild boars. Vet Parasitol 132(1–2):81–84
Morakote N, Khamboonruang C, Siriprasert V, Suphawitayanukul S, Marcanantachoti S, Thamasonthi W (1991) The value of enzyme-linked immunosorbent assay (ELISA) for diagnosis of human trichinosis. Trop Med Parasitol 42(3):172–174
Murrell KD, Pozio E (2011) Worldwide occurrence and impact of human trichinellosis, 1986–2009. Emerg Infect Dis 17(12):2194–2202
Ortega-Pierres MG, Yepez-Mulia L, Homan W, Gamble HR, Lim PL, Takahashi Y, Wassom DI, Appleton JA (1996) Workshop on a detailed characterization of Trichinella spiralis antigens: a platform for future studies on antigens and antibodies to this parasite. Parasite Immunol 18(6):273–284
Ren HJ, Cui J, Yang W, Liu RD, Wang ZQ (2013) Identification of differentially expressed genes of Trichinella spiralis larvae after exposure to host intestine milieu. PLoS One 8(6), e67570
Tang B, Liu M, Wang L, Yu S, Shi H, Boireau P, Cozma V, Wu X, Liu X (2015) Characterisation of a high-frequency gene encoding a strongly antigenic cystatin-like protein from Trichinella spiralis at its early invasion stage. Parasit Vectors 8:78
Wang ZQ, Cui J, Wu F, Mao FR, Jin XX (1998) Epidemiological, clinical and serological studies on trichinellosis in Henan Province, China. Acta Trop 71(3):255–268
Wang ZQ, Fu GY, Jing FJ, Jin J, Ren HJ, Jiang P, Cui J (2012) Detection of Trichinella spiralis circulating antigens in serum of experimentally infected mice by an IgY-mAb sandwich ELISA. Foodborne Pathog Dis 9(8):727–733
Wang L, Wang ZQ, Cui J (2013) Protein changes in Trichinella spiralis muscle larvae in vitro induced by bovine bile. Vet Parasitol 194(2–4):164–167
Wang L, Cui J, Hu DD, Liu RD, Wang ZQ (2014a) Identification of early diagnostic antigens from major excretory-secretory proteins of Trichinella spiralis muscle larvae using immunoproteomics. Parasit Vectors 7(1):40
Wang ZQ, Lin X, Zhang HW, Xu BL, Zhang X, Jiang P, Cui J (2014b) Serological survey for sparganum infection in people of central China. Helminthiologia 51(2):158–161
Yang W, Li LG, Liu RD, Sun GG, Liu CY, Zhang SB, Jiang P, Zhang X, Ren HJ, Wang ZQ, Cui J (2015) Molecular identification and characterization of Trichinella spiralis proteasome subunit beta type-7. Parasit Vectors 8:18
Yera H, Andiva S, Perret C, Limonne D, Boireau P, Dupouy-Camet J (2003) Development and evaluation of a Western blot kit for diagnosis of human trichinellosis. Clin Diagn Lab Immunol 10(5):793–796
Zhang D, Wang ZQ, Cui J (2003) Studies on common antigens among Trichinella spiralis, Paragonimus westermani and Clornorcis sinensis. J Trop Dis Parasitol 1(4):193–196
Zocevic A, Lacour SA, Mace P, Giovani B, Grasset-Chevillot A, Vallee I, Boireau P (2014) Primary characterization and assessment of a T. spiralis antigen for the detection of Trichinella infection in pigs. Vet Parasitol 205(3–4):558–567
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 81471981 and 81271860).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sun, G.G., Liu, R.D., Wang, Z.Q. et al. New diagnostic antigens for early trichinellosis: the excretory-secretory antigens of Trichinella spiralis intestinal infective larvae. Parasitol Res 114, 4637–4644 (2015). https://doi.org/10.1007/s00436-015-4709-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4709-3