Abstract
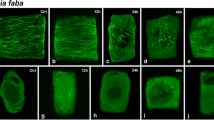
Distribution of post-translationally modified tubulins in cells of Nicotiana tabacum L. was analysed using a panel of specific antibodies. Polyglutamylated, tyrosinated, nontyrosinated, acetylated and Δ2-tubulin variants were detected on α-tubulin subunits; polyglutamylation was also found on β-tubulin subunits. Modified tubulins were detected by immunofluorescence microscopy in interphase microtubules, preprophase bands, mitotic spindles as well as in phragmoplasts. They were, however, located differently in the various microtubule structures. The antibodies against tyrosinated, acetylated and polyglutamylated tubulins gave uniform staining along all microtubules, while antibodies against nontyrosinated and Δ2-tubulin provided dotlike staining of interphase microtubules. Additionally, immunoreactivity of antibodies against acetylated and Δ2-tubulins was strong in the pole regions of mitotic spindles. High-resolution isoelectric focusing revealed 22 tubulin charge variants in N. tabacum suspension cells. Immunoblotting with antibodies TU-01 and TU-06 against conserved antigenic determinants of α- and β-tubulin molecules, respectively, revealed that 11 isoforms belonged to the α-subunit and 11 isoforms to the β-subunit. Whereas antibodies against polyglutamylated, tyrosinated and acetylated tubulins reacted with several α-tubulin isoforms, antibodies against nontyrosinated and Δ2-tubulin reacted with only one. The combined data demonstrate that plant tubulin is extensively posttranslationally modified and that these modifications participate in the generation of plant tubulin polymorphism.
Similar content being viewed by others
Abbreviations
- HRIF:
-
high-resolution isoelectric focusing
References
Andreu JM, Pereda JM (1993) Site-directed antibodies to tubulin. Cell Motil Cytoskel 26: 1–6
Åström H (1992) Acetylated α-tubulin in the pollen tube microtubules. Cell Biol Int Rep 16: 871–881
Boucher D, Larcher JC, Gros F, Denoulet P (1994) Polyglutamylation of tubulin as a progressive regulator of in vitro interactions between the microtubule-associated protein tau and tubulin. Biochemistry 33: 12471–12477
Bulinski JC, Gundersen GG (1991) Stabilization and post-translational modification of microtubules during cellular morphogenesis. BioEssays 13: 285–293
Dawson PJ, Lloyd CW (1985) Identification of multiple tubulins in taxol microtubules purified from carrot suspension cells. EMBO J 4: 2451–2455
Del Casino C, Li YQ, Moscatelli A, Tiezzi A, Cresti M (1993) Distribution of microtubules during the growth of tobacco pollen tubes. Biol Cell 79: 125–132
Dixon DC, Seagull RW, Triplett BA (1994) Changes in the accumulation of α- and β-tubulin isotypes during cotton fiber development. Plant Physiol 105: 1347–1353
Dráber P, Lagunowich LA, Dráberová E, Viklický V, Damjanov I (1988) Heterogeneity of tubulin epitopes in mouse fetal tissues. Histochemistry 89: 485–492
Dráber P, Dráberová E, Linhartová I, Viklický V (1989) Difference in the exposure of C- and N-terminal tubulin domains in cytoplasmic microtubules detected with domain-specific monoclonal antibodies. J Cell Sci 92: 519–528
Dráber P, Dráberová E, Viklický V (1991) Immunostaining of human spermatozoa with domain-specific monoclonal antibodies. Recognition of a unique beta-tubulin epitope in the sperm head. Histochemistry 95: 519–524
Dráberová E, Dráber P, Havlíček F, Viklický V (1986) A common antigenic determinant of vimentin and desmin defined by monoclonal antibody. Fol Biol (Praha) 32: 295–303
Ducket CM, Lloyd C (1994) Gibberellic acid-induced microtubule reorientation in dwarf peas is accompanied by rapid modification of an α-tubulin isotype. Plant J 5: 363–372
Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P (1990) Posttranslational glutamylation of α-tubulin. Science 247: 83–85
Eddé B, Rossier J, Le Caer J-P, Prome J-C, Desbruyères E, Gros F, Denoulet P (1992) Polyglutamylated α-tubulin can enter the tyrosination/detyrosination cycle. Biochemistry 31: 403–410
George JH, Misra L, Field DJ, Lee JC (1981) Polymorphism of brain tubulin. Biochemistry 20: 2402–2409
Geuens G, Gundersen GG, Nuydens R, Cornelissen F, Bulinski JC, DeBrabander M (1986) Ultrastructural colocalization of tyrosinated and detyrosinated α-tubulin in interphase and mitotic cells. J Cell Biol 103: 1883–1893
Goddard RH, Wick SM, Silflow CD, Snustad DP (1994) Microtubule components of plant cell cytoskeleton. Plant Physiol 104: 1–6
Gundersen GG, Kalnoski MH, Bulinski JC (1984) Distinct populations of microtubules: tyrosinated and nontyrosinated alpha-tubulin are distributed differently in vivo. Cell 38: 779–789
Hussey PJ, Snustad DP, Silflow CD (1991) Tubulin gene expression in higher plants. In: Lloyd CW (ed) The cytoskeletal basis of plant growth and form. Academic Press, London, pp 15–27
Kopczak SD, Haas NA, Hussey PJ, Silflow CD, Snustad DP (1992) The small genome of Arabidopsis contains at least six expressed α-tubulin genes. Plant Cell 4: 539–547
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
LeDizet M, Piperno G (1987) Identification of an acetylation site of Chlamydomonas α-tubulin. Proc Natl Acad Sci USA 84: 5720–5724
Linhartová I, Dráber P, Dráberová E, Viklický V (1992) Immunological discrimination of β-tubulin isoforms in developing mouse brain. Biochem J 288: 919–924
Linhartová I, Dráberová E, Viklický V, Dráber P (1993) Distribution of non-class-III β-tubulin isoforms in neuronal and nonneuronal cells. FEBS Lett 320: 79–82
Ludueña RF, Zimmermann HP, Little M (1988) Identification of the phosphorylated β-tubulin isotype in differentiated neuroblastoma cells. FEBS Lett 230: 142–146
Morejohn LC, Bureau TE, Tocchi LP, Fosket DE (1984) Tubulins from different higher plant species are immunologically nonidentical and bind colchicine differentially. Proc Natl Acad Sci USA 81: 1440–1444
Opatrný Z, Opatrná J (1976) The specificity of the effect of 2,4-D and NAA on the growth, micromorphology, and occurrence of starch in long-term Nicotiana tabacum L. cell strains. Biologia Plantarum (Praha) 18: 359–365
Paturle-Lafanechère L, Eddé B, Denoulet P, Van Dorsselaer A, Mazarguil H, Le Caer JP, Wehland J, Job D (1991) Characterization of a major tubulin variant which cannot be tyrosinated. Biochemistry 30: 10523–10528
Paturle-Lafanechère L, Manier M, Trigault N, Pirollet P, Mazarguil H, Job D (1994) Accumulation of delta 2-tubulin, a major tubulin variant that cannot be tyrosinated, in neuronal tissues and in stable microtubule assemblies. J Cell Sci 107: 1529–1543
Piperno G, Fuller MT (1985) Monoclonal antibodies specific for an acetylated form of α-tubulin recognise the antigen in cilia and flagella from variety of organisms. J Cell Biol 101: 2085–2094
Raybin D, Flavin M (1975) An enzyme tyrosilating alpha-tubulin and its role in microtubule assembly. Biochem Biophys Res Commun 65: 1088–1095
Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bré MH (1994) Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 266: 1688–1691
Snustad DP, Haas NA, Kopczak SD, Silflow CD (1992) The small genome of Arabidopsis contains at least nine expressed betatubulin genes. Plant Cell 4: 549–556
Webster DR, Borisy GG (1989) Microtubules are acetylated in domains that turn over slowly. J Cell Sci 92: 57–65
Wick SM, Seagull RW, Osborn M, Weber K, Gunning ES (1981) Immunofluorescence microscopy of organized microtubule arrays in structurally stabilized meristematic plant cells. J Cell Biol 89: 685–690
Wolff A, deNéchaud BD, Chillet D, Mazarguil H, Desbruyères E, Audebert S, Eddé B, Gros F, Denoulet P (1992) Distribution of glutamylated α- and β-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol 59: 425–432
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smertenko, A., Blume, Y., Viklický, V. et al. Post-translational modifications and multiple tubulin isoforms in Nicotiana tabacum L. cells. Planta 201, 349–358 (1997). https://doi.org/10.1007/s004250050077
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s004250050077