Summary
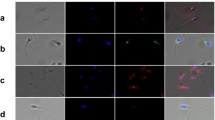
Four monoclonal antibodies that discriminate between structural domains of alpha-(TU-01, TU-04) or beta-(TU-06, TU-12) tubulin and a polyclonal anti-tubulin antibody were used for immunostaining of human spermatozoa using immunofluorescence microscopy. Specificity of antibodies was confirmed by immunoblotting experiments. Antibodies TU-01 and TU-06 uniformly stained the whole tail and the neck, whereas antibodies TU-04, TU-12 showed differential distribution of corresponding epitopes in the stable arrays of flagellar microtubules. Of the monoclonal antibodies used, only TU-12 against the antigenic determinant on C-terminal domain of β-tubulin showed strong reactivity with the equatorial segment of the head. The results document a differential exposure of tubulin epitopes at the single-cell level and suggest the existence of distinct tubulin populations in various structural compartments of the human spermatozoon.
Similar content being viewed by others
References
Amos LA, Klug A (1974) Arrangement of subunits in flagellar microtubules. J Cell Sci 14:523–549
Baccetti B, Burrini AG, Collodel G, Magnano AR, Piomboni P, Renieri T (1988) Immunocytochemistry and sperm pathology. J Submicrosc Cytol Pathol 20:209–224
Barros C, Franklin IE (1968) Behavior of gamete membranes during sperm entry into mammalian egg. J Cell Biol 37:C13-C18
Brandtzaeg P (1973) Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic exchange chromatography. Scand J Immunol 2 [Suppl]:273–290
Dráber P, Dráberová E, Linhartová I, Viklický V (1989) Differences in the exposure of C- and N-terminal tubulin domains in cytoplasmic microtubules detected with domain-specific monoclonal antibodies. J Cell Sci 92:519–528
Dráber P, Lagunowich LA, Dráberová E, Viklický V, Damjanov I (1988) Heterogeneity of tubulin epitopes in mouse fetal tissues. Histochemistry 89:485–492
Dráber P, Pokorná Z (1984) Differentiation antigens of mouse teratocarcinoma stem cells defined by monoclonal antibodies. Cell Differ 15:109–113
Dustin P (1984) Microtubules. Springer, Berlin Heidelberg New York
Fawcett DW (1975) The mammalian spermatozoon. Dev Biol 44:394–436
Friend DS (1982) Plasma-membrane diversity in a highly polarized cell. J Cell Biol 93:243–249
Fuller GM, Brinkley BR, Boughter JM (1975) Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science 187:948–950
Gallo JM, Escalier D, Schrével J, David G (1986) Differential distribution of tubulin epitopes in human spermatozoa. Eur J Cell Biol 40:111–116
Grimm M, Breitling F, Little M (1987) Location of the epitope for the α-tubulin monoclonal antibody TU-01. Biochim Biophys Acta 914:83–88
Jones HP, Bradford MM, McRorie RA, Cormier MJ (1978) High levels of calcium-dependent modulator protein in spermatozoa and its similarity to brain modulator protein. Biochem Biophys Res Commun 82:1264–1272
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lewis SA, Lee MGS, Cowan J (1985) Five mouse tubulin isotypes and their regulated expression during development. J Cell Biol 101:852–861
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mandelkow EM, Herrman M, Ruhl V (1985) Tubulin domains probed by limited proteolysis and subunit-specific antibodies. J Mol Biol 185:311–327
Peterson RN, Gillott M, Hunt W, Russell LD (1987) Organization of the boar spermatozoan plasma membrane: evidence for separate domains (subdomains) of integral membrane proteins in the plasma membrane overlying the principal segment of acrosome. J Cell Sci 88:343–349
Serrano L, Wandosell F, DeLa Torre J, Avila J (1986) Proteolytic modification of tubulin. Methods Enzymol 134:179–190
Serrano L, Diaz-Nido J, Wandosell F, Avila J (1987) Tubulin phosphorylation by casein kinase II is similar to that found in vivo. J Cell Biol 105:1731–1739
Shelanski ML, Gaskin F, Cantor CR (1973) Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci USA 70:765–768
Stanker LH, Vanderlaan M, Juarez-Salinas H (1985) One-step purification of mouse monoclonal antibodies from ascitic fluid by hydroxylaptite chromatography. J Immunol Methods 76:157–169
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Viklický V, Dráber P, Hašek J, Bártek J (1982) Production and characterization of a monoclonal antitubulin antibody. Cell Biol Int Rep 6:725–731
Virtanen I, Badley RA, Passivuo R, Lehto VP (1984a) Distinct cytoskeletal domains revealed in sperm cells. J Cell Biol 99:1083–1091
Virtanen I, Lehto VP, Kallajoki M, Blose SH (1984b) Differential localization of α- and β-tubulin in human sperm cells. J Cell Biol 99:41a
Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW (1975) A protein factor essential for microtubule assembly. Proc Natl Acad Sci USA 72:1858–1868
Wilkinson RF, Stanley HP, Bowman J (1975) The effect of vinblastine on spermiogenesis in Drosophila melanogaster: evidence for two functional classes of cytoplasmic microtubules. J Ultrastruct Res 53:354–365
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dráber, P., Dráberová, E. & Viklický, V. Immunostaining of human spermatozoa with tubulin domain-specific monoclonal antibodies. Recognition of a unique beta-tubulin epitope in the sperm head. Histochemistry 95, 519–524 (1991). https://doi.org/10.1007/BF00315749
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315749