Abstract
Main conclusion
Manipulation of sugar metabolism upon S. indica root colonization triggers changes in sugar pools and defense responses in A. thaliana.
Abstract
Serendipita indica is an endophytic fungus that establishes mutualistic relationships with many different plants including important crops as well as the model plant A. thaliana. Successful root colonization typically results in growth promotion and enhanced tolerance against various biotic and abiotic stresses. The fungus delivers phosphorus to the host and receives in exchange carbohydrates. There are hints that S. indica prefers hexoses, glucose, and fructose, products of saccharose cleavage driven by invertases (INVs) and sucrose synthases (SUSs). Carbohydrate metabolism in this interaction, however, remains still widely unexplored. Therefore, in this work, the sugar pools as well as the expression of SUSs and cytosolic INVs in plants colonized by S. indica were analyzed. Using sus1/2/3/4 and cinv1/2 mutants the importance of these genes for the induction of growth promotion and proper root colonization was demonstrated. Furthermore, the expression of several defense-related marker genes in both multiple mutants in comparison to the wild-type plants was determined. Our results show that in colonized A. thaliana plants S. indica manipulates the sugar metabolism by altering the expression of host’s INV and SUS and modulates both the sugar pools and plant defense in its favor. We conclude that the interaction A. thaliana–S. indica is a balancing act between cooperation and exploitation, in which sugar metabolism plays a crucial role. Small changes in this mechanism can lead to severe disruption resulting in the lack of growth promotion or altered colonization rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beneficial plant–microbe interactions evolve since 400 million years and play until today an important role for most plant species or even whole ecosystems (Parniske 2008). The best-known microbial group establishing such associations are arbuscular mycorrhizal fungi (AMF). During root colonization, these obligate symbionts facilitate uptake of important minerals—especially phosphorus and nitrogen (Luginbuehl and Oldroyd 2017). Further, they can confer tolerance and resistance to different abiotic stresses (drought, salt, toxins, or heavy metals) and to pathogenic organisms. In return, these fungi receive up to 20% of the plants fixed carbohydrates, mainly in form of hexoses (Parniske 2008).
Next to AMF, in the last years the focus of research moved to a group of Sebacinales with two phylogenetic subgroups: Sebacinaceae and Serendipitaceae (Weiß et al. 2011). Serendipitaceae contains several species with Serendipita indica (formerly Piriformospora indica) being the best studied member. This root endophyte was originally isolated in the Indian Thar Dessert from a spore of the AMF Funneliformis (= Glomus) mosseae (Verma et al. 1998). The fungus is able to establish mutualistic relationships with many different plants (Franken 2012), including the model plant Arabidopsis thaliana (Peskan-Berghöfer et al. 2004). A successful root colonization is divided in four different stages: (1) extracellular; (2) biotrophic; (3) cell death-associated and (4) fungal reproduction (Jacobs et al. 2011), leading to increased plant growth and development (reviewed in Franken 2012) and enhanced biotic and abiotic stress tolerance (reviewed in Gill et al. 2016).
Although the interaction between S. indica and its plant partners is widely considered as mutualistic (Zuccaro et al. 2011), after successful overcoming the first physical barriers the fungus must cope, similar to pathogens, with basic plant defense responses. To do that, the endophyte suppresses or manipulates these initial defense as described by Jacobs et al. (2011) and subsequently grows intracellularly. In this phase of colonization, the mutualistic nutritional exchange between S. indica and the host plant can be established. For that purpose, S. indica grows intracellularly and eventually forms coiled structures in root cells similar to arbuscules of AMF (Schäfer and Kogel 2009). In these structures, a symbiotic interface is formed in the apoplastic space that involves the infolding of the periarbuscular membrane, which is continuously aligned with the plant plasma membrane of the cortical cell surrounding the fungal wall (Felle et al. 2009). At this interface, the nutrient exchange of phosphate and carbohydrates occurs (Shachar-Hill et al. 1995; Karandashov and Bucher 2005). These carbohydrates constitute the cost demanded by the fungus for delivering phosphates to the host (Bonfante and Anca 2009). The mechanism related to the carbohydrate and phosphate exchange across the symbiotic interface is still unknown. In the past, it could only be assumed that among different carbohydrates endophytic fungi like S. indica prefer simple sugars, glucose, and fructose, and their uptake is generally similar to AMF. This hypothesis is supported, e.g. by the presence of monosaccharide transporters GpMST1 (Schüssler et al. 2006) and GspMST2 (Helber et al. 2011) in glomeromycotan fungi. Both transporters were shown to play a pivotal role during the symbiotic root colonization. Furthermore, the mutualistic fungus Phomopsis liquidambaris was shown to prioritize host hexoses in both, in vitro culture assays and in planta (Sun et al. 2020). Moreover, Schäfer et al. (2009) showed lower hexose and starch contents in S. indica colonized barley roots and the work of Zuccaro et al. (2011) delivered another evidence revealing 19 putative hexose transporter genes in S. indica genome. Lahrmann et al. (2013) showed up-regulation of some of them during colonization of A. thaliana roots. More recently, Rani et al. (2016) characterized PiHXT5 in more detail and showed its specific regulation during symbiosis with maize. Accordingly, Strehmel et al. (2016) made a confirming observation and demonstrated an increased concentration of different carbohydrates in A. thaliana roots colonized by S. indica.
In higher plants, sucrose is the main produced carbohydrate playing an important role in different biological processes (Salerno and Curatti 2003). It is transported through the phloem (Haritatos et al. 2000; Lemoine et al. 2013) and enters sink cells (e.g. roots) through plasmodesmata or the cell wall where it gets further processed (Wind et al. 2010). In A. thaliana, two types of transporter proteins, AtSUCs and AtSWEETs, actively facilitate this translocation (Durand et al. 2018). Once sucrose reaches its sink cell, sucrose synthases (SUSs), and invertases (INVs) split this disaccharide into its monomers glucose and fructose (Koch 2004). INVs cleave sucrose irreversibly, whereas SUSs produce fructose and UDP glucose, enabling reversible sucrose synthesis. In A. thaliana, six AtSUS (Baud et al. 2004; Bieniawska et al. 2007) and nine AtINV genes (Barratt et al. 2009; Barnes and Anderson 2018) are driving these reactions.
Besides their importance in plant nutrition, carbohydrates act as signaling molecules in many developmental processes as well as in defense responses (Wind et al. 2010). For instance, while sucrose mainly benefits cell differentiation and maturation, hexoses favor cell division and expansion (Koch 2004). Especially the role of sugar molecules in plant defense is recently getting increased attention (Bolouri Moghaddam and Van den Ende 2012; Tauzin and Giardina 2014; Trouvelot et al. 2014). In A. thaliana, Thibaud et al. (2004) showed increased AtPR-2 gene expression, when sucrose was added to the in vitro growth medium. Gebauer et al. (2017) could show that increased sugar levels in A. thaliana sweet11/sweet12 led to priming of the SA-pathway and therefore better protection against hemibiotroph fungus Colletotrichum higginsianum. These results are supported by work from Gómez-Ariza et al. (2007), who showed that higher sucrose levels in leaf tissue of transgenic rice plants trigger sucrose-dependent priming, resulting in a better protection against fungal and bacterial pathogens.
Recent reports indicate that S. indica takes up hexoses via functional transporters (Nizam et al. 2019). Accordingly, available sucrose in A. thaliana roots transported from the photosynthetic tissues must be further processed into hexoses. This can be achieved by shifts in the sugar pools and/or targeted manipulation of plant genes coding for sucrose-cleaving enzymes upon colonization. To test both possibilities, we performed sugar pool analyses of roots and shoots of S. indica-colonized plants. In the next step, we focused on host plant sucrose-cleaving enzymes, SUCs and CINVs and their expression during S. indica colonization of A. thaliana. Furthermore, to study whether these genes play an important role during successful colonization, we investigated quadruple mutant sus1/2/3/4 and double mutant cinv1/2 as well as analyzed the colonization rate and growth promotion of colonized plants. We also determined the expression of some important defense-related genes in both multiple mutants in comparison to the wild type plants. Our results demonstrate that in colonized A. thaliana plants, S. indica manipulates the sugar metabolism by altering the expression of host’s sucrose-processing enzymes and modulates both the sugar pools and plant defense clearly in its favor.
Materials and methods
Plant material and seed sterilization
All A. thaliana lines used in this work (Col-0 and T-DNA lines; Table 1) were kindly provided by Dr. Alison Smith (John Innes Centre, Norwich, UK). Sterilization of seeds was done according to Bohlmann and Wieczorek (2015). Briefly, seeds were surface-sterilized for 10 min in 5% Ca(OCl)2 with 0.1% Tween 20 followed by 5 min incubation in 70% ethanol and three subsequent washing steps in sterile dH2O.
Cultivation of A. thaliana
Plants were grown according to Sijmons et al. (1991) in sterile Petri dishes (9 cm diameter) on Knop medium in a culture room with a 16/8 light/dark photoperiod at 25 °C. Plates were prepared according to Cabello et al. (2014). Briefly, Knop medium of the shoot area (upper 1/3) contained 20 g L−1 sucrose (Knop+), whereas Knop medium of the root area (lower 2/3) contained no sucrose (Knop−). Each Petri dish contained on average 8–12 seeds. Petri dishes with plants harvested at earlier time point [3 day after inoculation (dai)] contained more seeds (12 seeds) than Petri dishes with plants harvested at later time points (7–14 dai: 8–10 seeds). Plates were sealed with parafilm and put into a culture room at the above-mentioned conditions.
S. indica cultivation and fungus inoculation
Serendipita indica was kindly provided by Prof. Ralf Oelmüller (Department of Plant Physiology, Matthias Schleiden Institute of Genetics, Bioinformatics and Molecular Botany, Friedrich-Schiller-University Jena, Jena, Germany). The fungus was stock-cultured on Käfer’s medium with adaptions from Johnson et al. (2011). Briefly, the fungus was precultured every four weeks by transferring fungal plugs on new Petri dishes containing Käfer’s medium. Every 6–12 months, the fungus was co-cultivated and re-isolated from A. thaliana roots. Experimental plates were inoculated with S. indica plugs of 6 mm diameter excised from 4-week-old S. indica culture grown on Käfer’s medium. Two plugs were placed top side down (for immediate contact with the new medium) in the root area next to the root tips. Control plates were inoculated with the same number of Käfer’s medium plugs without a fungus.
Harvesting and processing of plant material
Root and shoot material of colonized roots and shoots as well as their respective noncolonized controls were harvested 3, 7, and 14 dai. The collected material was immediately flash frozen in liquid nitrogen to prevent enzymatic or transcriptional alterations. Harvested plant material was ground with a Mixer Mill MM 400 (Retsch GmbH, Haan, Germany) for 1–3 min at 30 Hz into fine powder. Subsequently, the samples were stored at − 80 °C until further use.
Sugar pool analysis
Extraction
For carbohydrate extraction, roots and shoots of A. thaliana Col-0 colonized by S. indica as well as their noncolonized controls were harvested, ground, and stored as described above. Soluble carbohydrate extraction was performed according to Leach and Braun (2016) with adaptions from Srivastava et al. (2008). Briefly, samples were immersed in ice-cold MCW-extraction solution (methanol:chloroform:water, 12:5:3, by vol.). Samples consisted of 30–135 mg of fine ground frozen plant tissue. Subsequently, 10 × volume of ice-cold MCW extraction solution was added. Samples were vortexed and extraction was performed at 50 °C for 15 min using a thermomixer (Eppendorf, Hamburg, Germany). A total of three extraction steps were performed. The first extraction step contained 100 µM lactose, whereas the following extractions were performed using MCW extraction solution without lactose. The obtained extracts of each sample were pooled and 0.6 volumes of ddH2O were added to recover the soluble sugars in aqueous top phase.
Sample preparation and quantification
As recovered aqueous top phase may contain charged molecules which could interfere with ion chromatography (IC), collected top phase was pipetted through disposable chromatography column (BioRad, Hercules, CA, USA) containing cation exchange resin AG50W-X4 (BioRad), polyvinylpyrrolidone (PVPP, Acros Organics, Geel, Belgium), and anion exchange resin Amberlite IRA-67 free base (Merck, St. Louis, MO, USA) (Leach and Braun 2016). Briefly, 500 µL of AG50W-X4, 250 µL PVPP and 400 µL Amberlite IRA-67 free base slurries were added subsequently into disposable chromatography column. The resins were washed three times with ddH2O, before 1 mL of sample solution was added to remove strongly charged molecules. Finally, samples were taken up with 1 mL syringe (Merck) and filtered through 0.2 µm PVDF membrane (Roth, Karlsruhe, Germany). Samples were stored at − 20 °C, until they were sent for soluble sugar detection.
Carbohydrates were analyzed by IC coupled with pulsed amperometric detection on a Dionex™ ICS-5000 + System using an electrochemical detector with a gold electrode. Eluents were degassed by flushing with helium. An anion exchange 4 × 250-mm CarboPac PA1 column connected to a 4 × 50 mm guard column was used at 30 °C. The flow rate of the mobile phase was 1 mL min−1. A gradient with 150 mM NaOH (buffer A) and 0.5 M CH3COONa × 3 H2O in 150 mM NaOH (buffer B) was applied. Starting with 100% buffer A for 10 min then in 10 min to 50% buffer B; a wash step with 100% buffer B was applied for 5 min and subsequently 10 min 100% buffer A to equilibrate the column for the next sample (25 µL injection volume).
RNA isolation and cDNA synthesis
RNA extraction was done using RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). On-column DNA digestion was performed using RNase-Free DNase Set (Qiagen). All extraction steps were performed according to manufacturer’s instructions. RNA concentration and purity were assessed using Nano Drop 2000c (Thermo Fisher Scientific, Wilmington, DE, USA). Shoot samples were adjusted to 250 ng RNA µL−1 and directly colonized root samples to 100 ng RNA µL−1.
cDNA synthesis was done using Invitrogen SuperScript III reverse transcriptase (Thermo Fisher Scientific) according to manufacturer’s instructions. Briefly, 22 µL RNA, 2 µL dNTPs, 2 µL of hexa oligos and 2 µL of ddH2O were added together. The mixture was heated for 5 min at 65 °C followed by a 1 min incubation on ice. Subsequently, 8 µL 1st strand buffer, 2 µL 0.1 M DTT, 1 µL RiboLock RNase inhibitor (Thermo Fisher Scientific) and 1 µL reverse transcriptase was added to each sample followed by an incubation for 5 min at 25 °C. cDNA synthesis was performed for 60 min at 50 °C using a gradient Mastercycler (Eppendorf) followed by an inactivation step for 15 min at 75 °C. cDNA samples were stored at − 20 °C until further use.
DNA isolation
Colonized plant roots were harvested at 10 dai. Subsequently, root material was washed to get rid of fungal mycelium attached to the root surface and stored at − 80 °C until further use. Samples were aliquoted (≤ 100 mg) and ground with a Mixer Mill MM 400 according to above-mentioned instructions. DNA extraction of colonized root material was performed using DNeasy Plant Mini Kit (Qiagen) according to manufacturer’s instructions. Samples were stored at − 20 °C until further use.
Quantitative real-time PCR (qPCR)
qPCR was done using a peqSTAR 96Q Real-Time PCR Cycler (Peqlab Biotechnologie GmbH, Erlangen, Germany). Analysis was done using KAPA SYBR® FAST qPCR Master Mix (2 ×) kit (Merck) containing KAPA SYBR FAST DNA polymerase, reaction buffer, dNTPs, SYBR Green I dye, and MgCl2 at a final concentration of 2.5 mM according to the manufacturer’s descriptions. A master mix with gene-specific primers was prepared for every gene containing 7 µL ddH2O, 10 µL KAPA SYBR® FAST qPCR Master Mix (2 ×) kit, 0.5 µL of 10 mM 5′ primer and 0.5 µL of 10 mM 3′ primer. Eight genes coding for sucrose cleaving enzymes were analyzed: six sucrose synthases (AtSUS1, AtSUS2, AtSUS3, AtSUS4, AtSUS5, AtSUS6) and two cytosolic invertases (AtCINV1, AtCINV2). For plant defense, seven marker genes AtEIN3, AtERF1, AtPDF1.2, AtOXI1, AtACS6, AtPR3, and AtBI1 were analyzed. Colonization rate of S. indica was assessed using specific primer pair for SiTEF gene. AtUBP22 was used as an endogenous control according to Hofmann and Grundler (2007). All primer sequences are shown in the Supplementary Table S1. To each reaction well 2 µL of respective cDNA or DNA were added. A standard qPCR program consisted of an initial denaturation step (20 s at 95 °C) followed by 40 qPCR cycles (15 s at 95 °C and 20 s at 60 °C) and a melting stage.
The relative fold changes in gene expression were calculated based on the comparative CT method (Schmittgen and Livak 2008) using software peqSTAR96Q V2.1 (Peqlab Biotechnologie).
Determination of growth parameters
Arabidopsis thaliana Col-0 plants as well as single mutant lines (sus1-6 and cinv1-2) and multiple mutant lines (sus1/2/3/4 and cinv1/2) were analyzed at 10 dai. Shoot fresh and dry weight was determined using an analytical balance model 770 (Kern & Sohn GmbH, Balingen, Germany). For analysis of dry shoot weight, shoots were harvested and dried for 3 h at 90 °C in a UF 260 plus (Memmert GmbH, Schwabach, Germany). Main root length was assessed with a ruler, and the number of side roots per main root length was calculated.
Statistics
Statistical analysis was done using SPSS statistics software version 24.0 (Ehningen, Germany). qPCR-based gene expression study was performed in three independent biological repetitions (n = 3) with pooled plant material from 8–12 plants each. For colonization rate, qPCR tests were performed in eight independent biological repetitions (n = 8) with pooled plant material of 16–32 plants each. All measurements were performed with three technical replications. Differences in gene expression as compared to the respective control were assessed using Student’s t test (P < 0.05). Sugar pool analysis was performed in three independent repetitions (n = 3) with pooled plant material from 32–56 plants each. Differences between time points were assessed for each carbohydrate separately, using one-way ANOVA (Tukey’s test, P < 0.05). For fructose values in shoots of colonized plants, Welch ANOVA was performed (Dunnett-T3 test, P < 0.05). In the case of trehalose, due to missing data at 3 dai (2 of 3 measurements were below detection limit) the Student’s t test was performed (P < 0.05). Growth parameter experiments were performed in three independent biological repetitions with three samples each (n = 9). Each sample consisted of pooled plant material from three to ten plants. Outliers were removed with stem and leaf plot. Differences between colonized and noncolonized plants were assessed using two-way ANOVA (P < 0.05). When significant interaction was given, simple main effect analysis was performed (P < 0.01). In case of root length, variance of dependent variables was unequal (Levene test, P < 0.01) and the data of uncolonized cinv1/2 were not normally distributed (Shapiro–Wilk, P < 0.05).
Results
S. indica changes hosts sugar pools
In colonized roots sucrose levels decreased significantly over time, showing lower values at 7 dai and 14 dai (Fig. 1a). In contrast, fructose values increased significantly, showing higher levels at 7 dai and 14 dai (Fig. 1a). For glucose no significant changes were observed.
Sugar pool analysis of A. thaliana plants colonized by S. indica. a Sugar pools in colonized roots. b Sugar pools in shoots of colonized plants. Values indicate means ± SE of three independent repetitions from pooled plant material of 32–56 plantlets each. Means followed by a different letter indicate significant differences at P < 0.05 (Tukey’s test). In case of fructose values in shoots, Dunnett-T3 test was performed (P < 0.05). 1For trehalose and raffinose several samples were below detection limit of IC. In these cases, Student’s t test was performed, and asterisks indicate significant differences at P < 0.05
Trehalose was only detectable in colonized roots, showing significant accumulation over time (Fig. 1a). At 3 dai it could only be detected in one of three biological replicates, as its amount was at detection limit. Similar to trehalose, raffinose accumulated significantly over time with higher values in roots colonized by S. indica (Fig. 1a).
Shoots of plants colonized by S. indica showed no significant differences in sucrose, glucose and fructose values during first two time points (Fig. 1b). At 14 dai, however, glucose and fructose values increased significantly (Fig. 1b). Trehalose and raffinose amounts were only detectable at 14 dai.
To show differences between colonized and control samples, ratio of colonized to control samples was analyzed (Fig. 2). At 3 dai, no differences between colonized and noncolonized roots could be observed (Fig. 2a). In contrast, sucrose levels in colonized roots show significant lower values at 7 dai and 14 dai in comparison to noncolonized control roots (Fig. 2a).
Sugar pool ratio of A. thaliana plants colonized by S. indica to noncolonized control plants. a Sugar pool ratio of colonized roots to control roots. b Sugar pool ratio of colonized shoots to control shoots. Values indicate means ± SE of three independent repetitions. Asterisks indicate significant differences between colonized and control samples (Student’s t test; *at P < 0.05; **at P < 0.01). 1Trehalose and raffinose ratio is not determinable as several samples were below detection limit of IC
Furthermore, fructose levels in colonized roots increased significantly over time showing higher values at 7 dai in comparison to their noncolonized controls (Fig. 2a). At 14 dai only a trend in upregulation was observed. Glucose levels were similar at first two time points and showed no significant decrease in colonized roots at 14 dai when compared with noncolonized roots (Fig. 2a). In shoots, at 3 and 7 dai the changes in levels of all tested sugars were not significant. At 14 dai, levels of glucose and fructose were increased, however, these changes were not significant in comparison to the noncolonized controls (Fig. 2b).
S. indica modulates expression of hosts AtSUS and AtCINV genes
To test whether there is a correlation between sugar pools and the expression of AtSUS and AtCINV genes in shoots and roots of colonized A. thaliana plants, qPCR analyses were performed. These results demonstrate different spatio-temporal patterns of those genes (Fig. 3 and Supplementary Table S2). In colonized roots, significant upregulation of sucrose synthase genes was found for AtSUS4 at 3 dai and 7 dai, AtSUS1 at 7 dai as well as AtSUS3 at 14 dai. In shoots of colonized plants, significant downregulation of AtSUS5 at 7 dai was found, whereas AtSUS1 as well as AtSUS3 showed significant upregulation at 14 dai. In general, the changes in AtSUS expression were only moderate, nevertheless clear trends could be observed. In roots at all time points moderate upregulation of majority of AtSUSs is visible, whereas in shoots the downregulation of several AtSUSs at 3 and 7 dai was observed. Time point 14 dai for shoots falls slightly out and does not match the trend observed for both earlier time points. AtSUS1 and AtSUS3 show at this time point significant upregulation. In contrast to AtSUSs, cytosolic invertases were not affected by S. indica colonization, neither in colonized roots, nor in shoots of colonized plants.
sus1/2/3/4 and cinv1/2 do not exhibit S. indica-triggered growth promotion
To examine the importance of AtSUS and AtCINV genes for successful plant-S. indica interaction, growth parameters of A. thaliana mutant lines defective in multiple AtSUS and AtCINV upon S. indica colonization were analyzed. Preliminary tests of single mutants did not show significant changes when compared to the wild type (data not shown). In contrast, growth parameters in colonized sus1/2/3/4 and cinv1/2 showed distinctive changes (Table 2). In comparison to their noncolonized controls, both multiple mutant lines showed no changes in shoot fresh and dry weight whereas fresh and dry weight of colonized A. thaliana Col-0 in comparison to noncolonized control was significantly increased, 134% and 159%, respectively.
The length of the main root was unaffected in cinv1/2, whereas sus1/2/3/4 and A. thaliana Col-0 showed significant decrease, 76% and 83% respectively, when compared to the noncolonized controls (Table 2).
When concerning the number of side roots, no differences were found between colonized and noncolonized plants of line cinv1/2. In contrast, similar to the wild type the line sus1/2/3/4 showed an increased number of side roots, when compared with the noncolonized controls, 146% and 138%, respectively (Table 2).
Fungal colonization increased in cinv1/2
To reveal possible reasons for the lack of the typical growth promotion in sus1/2/3/4 and cinv1/2 lines upon S. indica colonization, we investigated the colonization rate in both mutants in comparison to colonized A. thaliana Col-0 using qPCR. Serendipita indica colonization was significantly increased in cinv1/2, whereas it remained unchanged in sus1/2/3/4 in comparison to the wild type (Fig. 4).
S. indica triggers changes in plant defense only in the wild type
To explain considerable differences in growth parameters and the colonization rate between mutant lines and the wild type we investigated several marker genes related to plant defense using qPCR. Significant upregulation of AtPR3, AtOXI1 and AtACS6 was observed in colonized A. thaliana wild type plants (Fig. 5). In contrast, sus1/2/3/4 showed no significant changes in expression of either gene, whereas cinv1/2 showed significant upregulation of AtPDF1.2 (Fig. 5).
qPCR analysis of plant defense-related genes in colonized roots of A. thaliana Col-0 as well as mutant lines sus1/2/3/4 and cinv1/2 compared with roots of their noncolonized controls at 7 dai. Shown are the means ± SE of three independent biological repetitions. Endogenous control: AtUBP22. Asterisks indicate significant differences in between colonized and noncolonized control roots (Student’s t test; *at P < 0.05; **at P < 0.01)
Enhanced expression of defense genes in noncolonized sus1/2/3/4 and cinv1/2
Noncolonized sus1/2/3/4 and cinv1/2 showed significant increase in expression of plant defense marker genes AtERF1 and AtPR3 when compared with noncolonized wild type. The expression of AtEIN3 was significantly increased only in noncolonized sus1/2/3/4 (Fig. 6a).
qPCR analysis of defense-related genes. a Noncolonized sus1/2/3/4 and cinv1/2 in comparison to uncolonized Col-0 at 7 dai. b Colonized sus1/2/3/4 and cinv1/2 in comparison to colonized Col-0 at 7 dai. Shown are the means ± SE of three independent biological repetitions. Endogenous control: AtUBP22. Asterisks indicate significant differences (Student’s t test, P < 0.05)
When gene expression of colonized sus1/2/3/4 and cinv1/2 was compared to colonized wild type, most of the genes showed no changes in their expression. Only AtOXI1 was significantly downregulated in cinv1/2 (Fig. 6b).
Discussion
Although many recent studies have been delivering single puzzle stones to the whole picture, the root colonization process of S. indica and related molecular mechanisms are still not fully understood. In this work, we’ve focused on the sugar metabolism during the mutualistic interaction between this endophyte and A. thaliana and analyzed sugar shifts and the role of plant AtINVs and AtSUSs as well as coherent defense responses in more details. The measured sugar pools as well as expression patterns of AtINV and AtSUS genes in root and shoot tissue of colonized and control A. thaliana plants support the hypothesis that S. indica remotely controls host’s sugar-related processes to boost its own carbohydrate supply, which is mainly based on monosaccharides (Parniske 2008; Rani et al. 2016). The colonization of A. thaliana is divided in three main phases as suggested by Jacobs et al. (2011). Hence, to correlate our experimental set-up to these phases, plant material was harvested at 3, 7, and 14 dai corresponding to biotrophic phase, cell death-associated phase and intracellular sporulation phase, respectively. Changes in gene expression were rather moderate, which might be due to the lack of sucrose in the plant growing medium (Angeles-Núñez and Tiessen 2012; Wang and Ruan 2013). However, we observed general downregulation of genes coding for AtSUS and AtCINV at 3 dai and 7 dai in shoots that could lead to lower processing of sucrose. This sucrose excess subsequently gets transported to roots colonized by S. indica. Such enhanced sugar transport to the infection site being a strong sink was previously also shown for phylloxera in grapevine roots (Griesser et al. 2015). In our study, however, no significantly increased sucrose pools were found in shoot tissue of colonized A. thaliana plants, which is rather striking. To explain this phenomenon, Bezrutczyk et al. (2018) proposed that sugar fluxes and pool sizes do not necessarily correlate due to increased uptake by endophytic fungi or plant pathogens.
As shown here, the upregulated plant AtSUSs in root tissue would finally perform cleavage of transported sucrose into the monomers glucose and fructose. Interestingly, two AtINVs tested here did not show any changes in expression in roots at all time points. Thus, it will be necessary to analyze the expression of other members of this gene family. However, in other host plants colonized by S. indica such as soybean, the upregulation of several INVs was recently demonstrated (Bajaj et al. 2018). In rice colonized by another mutualistic fungus, Phomopsis liquidambaris, activation of soluble acid invertases was shown, which resulted in enhanced hexose fluxes in colonized roots positively influencing fungal colonization (Sun et al. 2020). In the case of S. indica, this activation might be additionally enhanced/facilitated by elicitors released by the fungus (Vadassery et al. 2009; Jisha et al. 2018). Not only plant invertases are involved in this process. It is known that fungi secrete their own invertases to increase monosaccharide level. The role of these enzymes in the sugar metabolisms during plant–endophyte interaction should not be underestimated. For instance, genes for invertase and phosphoglucose-isomerase were found in genomes of S. indica and Serendipita herbamans and the corresponding protein activities have been detected in liquid culture of both fungi (Vincenzo De Rocchis, personal communication). Furthermore, invertase domains are significantly expanded in the genome of S. indica (Lahrmann et al. 2013). We also could show increased expression of two invertases from S. indica, PIIN_03242 and PIIN_08245, in colonized A. thaliana roots at different time points (data will be published elsewhere).
After the cleavage of sucrose by INVs and SUSs, monosaccharides can be taken up by S. indica using hexose transporters, whose high number was demonstrated in the S. indica genome (Zuccaro et al. 2009, 2011; Lahrmann et al. 2013). More recently, Rani et al. (2016) delivered a detailed functional characterization of PiHXT5 and showed that it is regulated in response to mutualism as well as glucose concentration. Hexose withdrawal is also supported by the significant lower sucrose levels in colonized roots as shown here, which fits the AtSUSs expression patterns. At the same time, fructose levels are significantly increased, whereas glucose levels remain constant. As PiHXT5 was indicated as high-affinity glucose transporter, and sucrose cleavage should lead to the formation of glucose and fructose in equimolar shares, measured carbohydrate pools support selective uptake of glucose. In shoots, downregulation of AtSUS and AtCINV at 3 and 7 dai was followed by upregulation at 14 dai. This might be due to the first signs of leaf chlorosis, although only green leaves were harvested for qPCR analysis. Nevertheless, the beginning of leaf senescence in those samples might be indicated by the upregulation of AtSUS3 (Bieniawska et al. 2007), increased hexose levels (Wingler et al. 2006) and the presence of trehalose-6-phosphate (T6P, Wingler 2018). Furthermore, lack of sugar in the growing medium together with the simultaneous endophyte colonization, which speeds up the plant development, might have caused and/or increased this effect (Wingler and Roitsch 2008). To summarize our results and hints from available literature, the putative sugar metabolism, sugar–phosphorus exchange and involvement of elicitors in S. indica-colonized A. thaliana plants are shown in the Fig. 7.
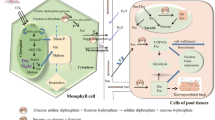
Putative sugar metabolism in A. thaliana during S. indica root colonization. a Colonization of root cortex cells via formation of intracellularly growing hypha. During this process coiled structures are formed, which ensure sophisticated interaction between S. indica and the host plant. In shoots, sugar pools are not affected by root S. indica colonization, however, increased flux of sucrose into roots might occur (Bezrutczyk et al. 2018). Interestingly, in S. indica-colonized roots only fructose amount increases significantly, whereas glucose level remains unchanged due to increased glucose uptake by S. indica. b Schematic overview of symbiotic interface formed during S. indica root colonization. In plant cytosol, there is increased cleavage of sucrose driven by plant invertases (AtINV) and sucrose synthases (AtSUS). This reaction might be enhanced/facilitated by elicitors released by S. indica (yellow dots; Vadassery et al. 2009; Jisha et al. 2018). In addition, in apoplast secreted fungal invertases (SiINV) lead to enhanced hexose level. Subsequently, plant SUC and SWEET transporters translocate available carbohydrates into apoplast, where remaining sucrose might be cleaved by invertases released by S. indica (red arrow, Vincenzo De Rocchis, personal communication; Lahrmann et al. 2013). Finally, glucose is taken up by S. indica selectively via e.g. high affinity transporter PiHXT5 (Rani et al. 2016). In exchange for this service, the root endophyte shifts inorganic phosphorus (black dots) into apoplast (Yadav et al. 2010), from where the host plant takes it up via members of phosphate transporter family PHT1 located in the plasma membrane (Nussaume et al. 2011). Adobe Illustrator was used to create the artwork
Sucrose and its cleavage monosaccharides might not be only nutrients for S. indica but they might also play other roles in establishing the mutualistic relationship. There are several other aspects and functions of sucrose in plants that need to be taken into account (reviewed in Morkunas and Ratajczak 2014). For instance, sucrose functions as a hormone-like signaling molecule (Lou et al. 2007) and together with glucose and fructose induces sugar-specific changes in the gene expression (Kunz et al. 2014). This is the case in S. indica-colonized plants where downregulation of defense-related genes at early stages of colonization enables the plant–endophyte beneficial interaction (Schäfer et al. 2009; Camehl et al. 2011; Jacobs et al. 2011; Vahabi et al. 2015). In addition, UDP glucose is discussed to be an extracellular signaling molecule, which may even be perceived as damage-associated molecular pattern (Janse van Rensburg and Van den Ende 2017). Sucrose also plays a role in the induction of anthocyanin biosynthesis (Solfanelli et al. 2006; Das et al. 2012), which was shown in plants colonized by different fungal endophytes (Yu et al. 2020; Bu et al. 2020), and promotes phenylpropanoid biosynthesis (Ferri et al. 2011), which was shown in plants colonized by the endophyte Gilmaniella sp. (Yuan et al. 2019). T6P is both, a signal molecule and negative feedback regulator of sucrose in A. thaliana (Figueroa and Lunn 2016). There is a connection between trehalose formation and cell wall thickening as well as cell division (Gómez et al. 2006). Glucose has an important role as signal molecule interacting with CINV1 regulating A. thaliana root growth (Meng et al. 2020). Concerning all this, it is possible that at the early stage of the S. indica–host interaction two different processes take place. On the one hand, plant starts to defend itself, i.e., changing its sugar status that precisely modulates different defense responses (reviewed in Morkunas and Ratajczak 2014) and on the other hand S. indica actively manipulates the plant sugar levels in order to suppress these defense responses and to receive sufficient amounts of desired carbohydrates. This is in line with other studies showing a number of other plant pathogens or symbionts including some endophytic (e.g. Glomus intraradices; Schaarschmidt et al. 2007) and biotrophic fungi (e.g. Blumeria gramini; Scholes et al. 1994; Wright et al. 1995; Swarbrick et al. 2006), which change the sugar content of their hosts for their own benefit (reviewed in Tauzin and Giardina 2014).
We demonstrated that S. indica root colonization severely alters sugar pools and moderately changes the expression of AtINV and AtSUS genes in host tissues. In the next step we tested whether mutations in those genes have an impact on the host growth promotion triggered typically by S. indica colonization. A. thaliana Col-0 colonized by S. indica showed typical growth promoting effects, which is in line with available data (Peskan-Berghöfer et al. 2004). Similarly, we could see growth increase in single inv and sus mutants. Subsequently, we decided to test multiple mutants cinv1/2 and sus1/2/3/4. According to Barratt et al. (2009), growth phenotype of the mutant line cinv1/2 fits general carbon starvation due to reduced sucrose catabolism in roots. In comparison, mutant line sus1/2/3/4 shows no differences in starch or sugar content and grows normally. In contrast to single mutant lines, in both multiple mutants, we did not detect any growth promotion, which might be due to disturbed sugar metabolism and signaling in both lines (reviewed in Bolouri Moghaddam and Van den Ende 2012; Trouvelot et al. 2014). This unsuccessful root endophyte–host interaction might be a result of nonfunctional diminishment of plant defense responses. Therefore, in the next step we analyzed the colonization rate of both multiple mutants. Interestingly, we found increased fungal colonization rate only in cinv1/2 and not in sus1/2/3/4. This raises the question what might cause that? Barratt et al. (2009) demonstrated decreased invertase activity in cinv1/2, which might be the reason for altered S. indica root colonization. At least for AMF, Schaarschmidt et al. (2007) showed correlation between invertase activity in Nicotiana tabacum leaves and the degree of root mycorrhization by Glomus intraradices. For S. indica further research is required to shed more light on this phenomenon.
Another reason for enhanced colonization rate in cinv1/2 could be altered defense responses in this mutant due to impaired sugar metabolism and signaling. Therefore, we tested expression of several defense-related genes in both mutants. Interestingly, when we compared colonized sus1/2/3/4 and cinv1/2 with their respective controls, the expression of these genes was mainly unaffected. This analysis however could not exclude if in noncolonized mutant lines defense was already altered prior to root endophyte colonization. Therefore, gene expression of noncolonized and colonized mutants was compared to uncolonized and colonized wild type plants, respectively. For the first combination we obtained increased expression of several defense-related genes (e.g. in sus1/2/3/4 AtEIN3, AtERF1 and AtPR3; in cinv1/2 AtERF1 and AtPR3). Thus, this finding strengthens the hypothesis that hampered processing of carbohydrates in these mutants have an impact on plant defense. In addition, comparing colonized wild type and colonized sus1/2/3/4 and cinv1/2 we did not observe much changes in gene expression. However, colonized roots of cinv1/2 exhibited significant lower expression of OXI1 (Oxidative signal inducible1) in comparison to the colonized wild type. Camehl et al. (2011) analyzed the role of OXI1 and PDK1 (3-phosphoinositide-dependent protein kinase1), a kinase that regulates OXI1, in growth promotion in response to S. indica colonization. The authors demonstrated that OXI1 expression is upregulated in S. indica-colonized roots and that growth response is mediated by a cascade consisting of PDK1 and OXI1. Thus, significantly decreased OXI1 expression in cinv1/2 might be the reason for both, higher colonization rate and the lack of typical growth promotion. Moreover, this protein kinase regulates both basal resistance (pattern associated molecular patterns = PAMP) and effector triggered immunity (ETI) in response to the bacterial pathogen Pseudomonas syringae (Petersen et al. 2009). The authors reported on the importance of OXI1 for immunity of A. thaliana against P. syringae and showed that both overexpression as well as down-regulation of OXI1 can lead to a higher susceptibility against biotrophic pathogens. Hence, this finding delivers an additional possible explanation for increased accommodation of S. indica in cinv1/2 roots. In roots of colonized sus1/2/3/4 no downregulation of OXI1 could be observed in comparison to the wild type. This explains similar colonization rate to the rate observed in the wild type roots.
Our results demonstrate that S. indica manipulates the sugar metabolism in order to change the sugar status as well as modulates the defense responses in A. thaliana. Thus, we postulate that in this interaction sugar is not only a nutrient for a fungus, but it also plays an important role in the regulation of defense responses, which is pivotal for the establishment of the mutualistic relationship. We showed that sugars are affecting the successful root colonization and further growth of S. indica, which might indicate the role of sugars as important signal molecules what has been recently postulated. This study shows that the interaction A. thaliana–S. indica is a balancing act between cooperation and exploitation, in which sugar metabolism plays a crucial role. Small changes in this mechanism can lead to severe disruption resulting in the lack of growth promotion or altered colonization rate.
Author contributions Statement
MO: performed and conceived experiments, analyzed the data and designed illustrations; CL and RL: performed IC for sugar pool analysis; RD: conceived parts of the research; MO and KW: wrote the manuscript; SS: contributed with critical manuscript revisions; KW: conceived and designed the research. All authors read and approved the final manuscript.
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- dai:
-
Days after inoculation
- (c)INV:
-
(Cytosolic)invertase
- SUS:
-
Sucrose synthase
- sus :
-
SUS knock-down mutant
- inv :
-
INV knock-down mutant
References
Angeles-Núñez JG, Tiessen A (2012) Regulation of AtSUS2 and AtSUS3 by glucose and the transcription factor LEC2 in different tissues and at different stages of Arabidopsis seed development. Plant Mol Biol 78:377–392. https://doi.org/10.1007/s11103-011-9871-0
Bajaj R, Huang Y, Gebrechristos S, Mikolajczyk B, Brown H, Prasad R, Varma A, Bushley KE (2018) Transcriptional responses of soybean roots to colonization with the root endophytic fungus Piriformospora indica reveals altered phenylpropanoid and secondary metabolism. Sci Rep 8:10227. https://doi.org/10.1038/s41598-018-26809-3
Barnes WJ, Anderson CT (2018) Cytosolic invertases contribute to cellulose biosynthesis and influence carbon partitioning in seedlings of Arabidopsis thaliana. Plant J 94:956–974. https://doi.org/10.1111/tpj.13909
Barratt DH, Derbyshire P, Findlay K, Pike M, Wellner N, Lunn J, Feil R, Simpson C, Maule AJ, Smith AM (2009) Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc Natl Acad Sci USA 106:13124–13129. https://doi.org/10.1073/pnas.0900689106
Baud S, Vaultier MN, Rochat C (2004) Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J Exp Bot 55:397–409. https://doi.org/10.1093/jxb/erh047
Bezrutczyk M, Yang J, Eom JS, Prior M, Sosso D, Hartwig T, Szurek B, Oliva R, Vera-Cruz C, White FF, Yang B, Frommer WB (2018) Sugar flux and signaling in plant–microbe interactions. Plant J 93:675–685. https://doi.org/10.1111/tpj.13775
Bieniawska Z, Paul Barratt DH, Garlick AP, Thole V, Kruger NJ, Martin C, Zrenner R, Smith AM (2007) Analysis of the sucrose synthase gene family in Arabidopsis. Plant J 49:810–828. https://doi.org/10.1111/j.1365-313X.2006.03011.x
Bohlmann H, Wieczorek K (2015) Infection assay of cyst nematodes on Arabidopsis roots. Bio-protocol 5:e1596. https://doi.org/10.21769/BioProtoc.1596
Bolouri Moghaddam MR, Van den Ende W (2012) Sugars and plant innate immunity. J Exp Bot 63:3989–3998. https://doi.org/10.1093/jxb/ers129
Bonfante P, Anca IA (2009) Plants, mycorrhizal fungi, and bacteria: a network of interactions. Annu Rev Microbiol 63:363–383. https://doi.org/10.1146/annurev.micro.091208.073504
Bu C, Zhang Q, Zeng J, Cao X, Hao Z, Qiao D, Cao Y, Xu H (2020) Identification of a novel anthocyanin synthesis pathway in the fungus Aspergillus sydowii H-1. BMC Genom 21:29. https://doi.org/10.1186/s12864-019-6442-2
Cabello S, Lorenz C, Crespo S, Cabrera J, Ludwig R, Escobar C, Hofmann J (2014) Altered sucrose synthase and invertase expression affects the local and systemic sugar metabolism of nematode-infected Arabidopsis thaliana plants. J Exp Bot 65:201–212. https://doi.org/10.1093/jxb/ert359
Camehl I, Drzewiecki C, Vadassery J, Shahollari B, Sherameti I, Forzani C, Munnik T, Hirt H, Oelmüller R (2011) The OXI1 kinase pathway mediates Piriformospora indica-induced growth promotion in Arabidopsis. PLoS Pathog 7:e1002051. https://doi.org/10.1371/journal.ppat.1002051
Das PK, Shin DH, Choi SB, Park YI (2012) Sugar-hormone cross-talk in anthocyanin biosynthesis. Mol Cells 34:501–507. https://doi.org/10.1007/s10059-012-0151-x
Durand M, Mainson D, Porcheron B, Maurousset L, Lemoine R, Pourtau N (2018) Carbon source–sink relationship in Arabidopsis thaliana: the role of sucrose transporters. Planta 247:587–611. https://doi.org/10.1007/s00425-017-2807-4
Felle HH, Waller F, Molitor A, Kogel KH (2009) The mycorrhiza fungus Piriformospora indica induces fast root-surface pH signaling and primes systemic alkalinization of the leaf apoplast upon powdery mildew infection. Mol Plant Microbe Interact 22:1179–1185. https://doi.org/10.1094/MPMI-22-9-1179
Ferri M, Righetti L, Tassoni A (2011) Increasing sucrose concentrations promote phenylpropanoid biosynthesis in grapevine cell cultures. J Plant Physiol 168:189–195. https://doi.org/10.1016/j.jplph.2010.06.027
Figueroa CM, Lunn JE (2016) A tale of two sugars: trehalose 6-phosphate and sucrose. Plant Physiol 172:7–27. https://doi.org/10.1104/pp.16.00417
Franken P (2012) The plant strengthening root endophyte Piriformospora indica: potential application and the biology behind. Appl Microbiol Biotechnol 96:1455–1464. https://doi.org/10.1007/s00253-012-4506-1
Gebauer P, Korn M, Engelsdorf T, Sonnewald U, Koch C, Voll LM (2017) Sugar accumulation in leaves of Arabidopsis sweet11/sweet12 double mutants enhances priming of the salicylic acid-mediated defense response. Front Plant Sci 8:1378. https://doi.org/10.3389/fpls.2017.01378
Gill SS, Gill R, Trivedi DK, Anjum NA, Sharma KK, Ansari MW, Ansari AA, Johri AK, Prasad R, Pereira E, Varma A, Tuteja N (2016) Piriformospora indica: potential and significance in plant stress tolerance. Front Microbiol 7:332. https://doi.org/10.3389/fmicb.2016.00332
Gómez LD, Baud S, Gilday A, Li Y, Graham IA (2006) Delayed embryo development in the ARABIDOPSIS TREHALOSE-6-PHOSPHATE SYNTHASE 1 mutant is associated with altered cell wall structure, decreased cell division and starch accumulation. Plant J 46:69–84. https://doi.org/10.1111/j.1365-313X.2006.02662.x
Gómez-Ariza J, Campo S, Rufat M, Estopà M, Messeguer J, San Segundo B, Coca M (2007) Sucrose-mediated priming of plant defense responses and broad-spectrum disease resistance by overexpression of the maize pathogenesis-related PRms protein in rice plants. Mol Plant Microbe Interact 20:832–842. https://doi.org/10.1094/MPMI-20-7-0832
Griesser M, Lawo NC, Crespo-MartinezS S-H, Wieczorek K, Gorecka M, Liebner F, Zweckmair T, Stralis Pavese N, Kreil D, Forneck A (2015) Phylloxera (Daktulosphaira vitifoliae Fitch) alters the carbohydrate metabolism in root galls to allowing the compatible interaction with grapevine (Vitis ssp.) roots. Plant Sci 234:38–49. https://doi.org/10.1016/j.plantsci.2015.02.002
Haritatos E, Medville R, Turgeon R (2000) Minor vein structure and sugar transport in Arabidopsis thaliana. Planta 211:105–111. https://doi.org/10.1007/s004250000268
Helber N, Wippel K, Sauer N, Schaarschmidt S, Hause B, Requena N (2011) A versatile monosaccharide transporter that operates in the arbuscular mycorrhizal fungus Glomus sp. is crucial for the symbiotic relationship with plants. Plant Cell 23:3812–3823. https://doi.org/10.1105/tpc.111.089813
Hofmann J, Grundler FMW (2007) Identification of reference genes for qRT-PCR studies of gene expression in giant cells and syncytia induced in Arabidopsis thaliana by Meloidogyne incognita and Heterodera schachtii. Nematology 9:317–323. https://doi.org/10.1163/156854107781352034
Jacobs S, Zechmann B, Molitor A, Trujillo M, Petutschnig E, Lipka V, Kogel KH, Schäfer P (2011) Broad-spectrum suppression of innate immunity is required for colonization of Arabidopsis roots by the fungus Piriformospora indica. Plant Physiol 156:726–740. https://doi.org/10.1104/pp.111.176446
Jisha S, Gouri PR, Anith KN, Sabu KK (2018) Piriformospora indica cell wall extract as the best elicitor for asiaticoside production in Centella asiatica (L.) urban, evidenced by morphological, physiological and molecular analyses. Plant Physiol Biochem 125:106–115. https://doi.org/10.1016/j.plaphy.2018.01.021
Johnson JM, Sherameti I, Ludwig A, Nongbri PL, Sun C, Lou B, Varma A, Oelmüller R (2011) Protocols for Arabidopsis thaliana and Piriformospora indica co-cultivation—a model system to study plant beneficial traits. Endocyt Cell Res 21:101–113
Karandashov V, Bucher M (2005) Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci 10:22–29. https://doi.org/10.1016/j.tplants.2004.12.003
Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7:235–246. https://doi.org/10.1016/j.pbi.2004.03.014
Kunz S, Pesquet E, Kleczkowski LA (2014) Functional dissection of sugar signals affecting gene expression in Arabidopsis thaliana. PLoS ONE 9:e100312. https://doi.org/10.1371/journal.pone.0100312
Lahrmann U, Ding Y, Banhara A, Rath M, Hajirezaei MR, Döhlemann S, von Wirén N (2013) Host-related metabolic cues affect colonization strategies of a root endophyte. Proc Natl Acad Sci USA 110:13965–13970. https://doi.org/10.1073/pnas.1301653110
Leach KA, Braun DM (2016) Soluble sugar and starch extraction and quantification from maize (Zea mays) leaves. Curr Protoc Plant Biol 1:139–161. https://doi.org/10.1002/cppb.20018
Lemoine R, Camera SL, Atanassova R, Dédaldéchamp F, Allario T, Pourtau N, Bonnemain J, Laloi M, Coutos- Thévenot P, Maurousset L, Faucher M, Girousse C, Lemonnier P, Parrilla J, Durand M (2013) Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci 4:272. https://doi.org/10.3389/fpls.2013.00272
Lou Y, Gou JY, Xue HW (2007) PIP5K9, an Arabidopsis phosphatidylinositol monophosphate kinase, interacts with a cytosolic invertase to negatively regulate sugar-mediated root growth. Plant Cell 19:163–181. https://doi.org/10.1105/tpc.106.045658
Luginbuehl LH, Oldroyd GED (2017) Understanding the arbuscule at the heart of endomycorrhizal symbioses in plants. Curr Biol 27:R952–R963. https://doi.org/10.1016/j.cub.2017.06.042
Meng LS, Wei ZQ, Cao XY, Tong C, Lv MJ, Yu F, Loake GJ (2020) Cytosolic invertase-mediated root growth is feedback-regulated by a glucose-dependent signaling loop. Plant Physiol 184:778. https://doi.org/10.1104/pp.20.00778
Morkunas I, Ratajczak L (2014) The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiol Plant 36:1607–1619. https://doi.org/10.1007/s11738-014-1559-z
Nizam S, Qiang X, Wawra S, Nostadt R, Getzke F, Schwanke F, Dreyer I, Langen G, Zuccaro A (2019) Serendipita indica E5’NT modulates extracellular nucleotide levels in the plant apoplast and affects fungal colonization. EMBO Rep 20:e47430. https://doi.org/10.15252/embr.201847430
Nussaume L, Kanno S, Javot H, Marin E, Pochon N, Ayadi A, Nakanishi TM, Thibaud MC (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci 2:83. https://doi.org/10.3389/fpls.2011.00083
Parniske M (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6:763–775. https://doi.org/10.1038/nrmicro1987
Peskan-Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Kost G, Varma A, Oelmüller R (2004) Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant–microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant 122:465–477. https://doi.org/10.1111/j.1399-3054.2004.00424.x
Petersen LN, Ingle RA, Knight MR, Denby KJ (2009) OXI1 protein kinase is required for plant immunity against Pseudomonas syringae in Arabidopsis. J Exp Bot 60:3727–3735. https://doi.org/10.1093/jxb/erp219
Rani M, Raj S, Dayaman V, Kumar M, Dua M, Johri AK (2016) Functional characterization of a hexose transporter from root endophyte Piriformospora indica. Front Microbiol 7:1083. https://doi.org/10.3389/fmicb.2016.01083
Salerno GL, Curatti L (2003) Origin of sucrose metabolism in higher plants: when, how and why? Trends Plant Sci 8:63–69. https://doi.org/10.1016/S1360-1385(02)00029-8
Schaarschmidt S, Kopka J, Ludwig-Müller J, Hause B (2007) Regulation of arbuscular mycorrhization by apoplastic invertases: enhanced invertase activity in the leaf apoplast affects the symbiotic interaction. Plant J 51:390–405. https://doi.org/10.1111/j.1365-313X.2007.03150.x
Schäfer P, Kogel KH (2009) The sebacinoid fungus Piriformospora indica: an orchid mycorrhiza which may increase host plant reproduction and fitness. In: Deising H (ed) Plant relationships. The Mycota V, 2nd edn. Springer, Berlin, pp 99–102
Schäfer P, Pfiffi S, Voll LM, Zajic D, Chandler PM, Waller F, Scholz U, Pons-Kühnemann J, Sonnewald S, Sonnewald U, Kogel KH (2009) Manipulation of plant innate immunity and gibberellin as factor of compatibility in the mutualistic association of barley roots with Piriformospora indica. Plant J 59:461–474. https://doi.org/10.1111/j.1365-313X.2009.03887.x
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. https://doi.org/10.1038/nprot.2008.73
Scholes JD, Lee PJ, Horton P, Lewis DH (1994) Invertase: understanding changes in the photosynthetic and carbohydrate metabolism of barley leaves infected with powdery mildew. New Phytol 126:213–222. https://doi.org/10.1111/j.1469-8137.1994.tb03939.x
Schüssler A, Martin H, Cohen D, Fitz M, Wipf D (2006) Characterization of a carbohydrate transporter from symbiotic glomeromycotan fungi. Nature 444:933–936. https://doi.org/10.1038/nature05364
Shachar-Hill Y, Pfeffer PE, Douds D, Osman SF, Doner LW, Ratcliffe RG (1995) Partitioning of intermediary carbon metabolism in vesicular–arbuscular mycorrhizal leek. Plant Physiol 108:7–15. https://doi.org/10.1104/pp.108.1.7
Sijmons PC, Grundler FMW, Nv M, Burrows PR, Wyss U (1991) Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J 1:245–254. https://doi.org/10.1111/j.1365-313x.1991.00245.x
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646. https://doi.org/10.1104/pp.105.072579
Srivastava AC, Ganesan S, Ismail IO, Ayre BG (2008) Functional characterization of the Arabidopsis AtSUC2 sucrose/H+ symporter by tissue-specific complementation reveals an essential role in phloem loading but not in long-distance transport. Plant Physiol 148:200–211. https://doi.org/10.1104/pp.108.124776
Strehmel N, Mönchgesang S, Herklotz S, Drüger S, Ziegler J, Scheel D (2016) Piriformospora indica stimulates root metabolism of Arabidopsis thaliana. Int J Mol Sci 17:1091. https://doi.org/10.3390/ijms17071091
Sun K, Zhang W, Yuan J, Song SL, Wu H, Tang MJ, Xu FJ, Xie XG, Dai CC (2020) Nitrogen fertilizer-regulated plant–fungi interaction is related to root invertase-induced hexose generation. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiaa139
Swarbrick PJ, Schulze-Lefert P, Scholes JD (2006) Metabolic consequences of susceptibility and resistance (race-specific and broad-spectrum) in barley leaves challenged with powdery mildew. Plant Cell Environ 29:1061–1076. https://doi.org/10.1111/j.1365-3040.2005.01472.x
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5:293. https://doi.org/10.3389/fpls.2014.00293
Thibaud MC, Gineste S, Nussaume L, Robaglia C (2004) Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. Plant Physiol Biochem 42:81–88. https://doi.org/10.1016/j.plaphy.2003.10.012
Trouvelot S, Héloir MC, Poinssot B, Gauthier A, Paris F, Guillier C, Combier M, Trdá L, Daire X, Adrian M (2014) Carbohydrates in plant immunity and plant protection: roles and potential application as foliar sprays. Front Plant Sci 5:592. https://doi.org/10.3389/fpls.2014.00592
Vadassery J, Ranf S, Drzewiecki C, Mithöfer A, Mazars C, Scheel D, Lee J, Oelmüller R (2009) A cell wall extract from the endophytic fungus Piriformospora indica promotes growth of Arabidopsis seedlings and induces intracellular calcium elevation in roots. Plant J 59:193–206. https://doi.org/10.1111/j.1365-313X.2009.03867.x
Vahabi K, Sherameti I, Bakshi M, Mrozinska A, Ludwig A, Reichelt M, Oelmüller R (2015) The interaction of Arabidopsis with Piriformospora indica shifts from initial transient stress induced by fungus-released chemical mediators to a mutualistic interaction after physical contact of the two symbionts. BMC Plant Biol 15:58. https://doi.org/10.1186/s12870-015-0419-3
van Janse Rensburg HC, Van den Ende W (2017) UDP-glucose: a potential signaling molecule in plants? Front Plant Sci 8:2230. https://doi.org/10.3389/fpls.2017.02230
Verma S, Verma A, Rexer K, Hassel A, Kost G, Sarbhoy A, Bisen P, Bütenhorn B, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90:896–903. https://doi.org/10.2307/3761331
Wang L, Ruan YL (2013) Regulation of cell division and expansion by sugar and auxin signaling. Front Plant Sci 4:163. https://doi.org/10.3389/fpls.2013.00163
Weiß M, Sýkorová Z, Garnica S, Riess K, Martos F, Krause C, Oberwinkler F, Bauer R, Redecker D (2011) Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PLoS ONE 6:e16793. https://doi.org/10.1371/journal.pone.0016793
Wind J, Smeekens S, Hanson J (2010) Sucrose: metabolite and signaling molecule. Phytochemistry 71:1610–1614. https://doi.org/10.1016/j.phytochem.2010.07.007
Wingler A (2018) Transitioning to the next phase: the role of sugar signaling throughout the plant life cycle. Plant Physiol 176:1075–1084. https://doi.org/10.1104/pp.17.01229
Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol 10:50–62. https://doi.org/10.1111/j.1438-8677.2008.00086.x
Wingler A, Purdy S, MacLean JA, Pourtau N (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57:391–399. https://doi.org/10.1093/jxb/eri279
Wright DP, Baldwin BC, Shephard MC, Scholes JD (1995) Source–sink relationships in wheat leaves infected with powdery mildew. I. Alterations in carbohydrate metabolism. Physiol Mol Plant Pathol 47:237–253. https://doi.org/10.1006/pmpp.1995.1055
Yadav V, Kumar M, Deep DK, Kumar H, Sharma R, Tripathi T, Tuteja N, Saxena AK, Johri AK (2010) A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J Biol Chem 285:26532–26544. https://doi.org/10.1074/jbc.M110.111021
Yu M, Chen JC, Qu JZ, Liu F, Zhou M, Ma YM, Xiang SY, Pan XX, Zhang HB, Yang MZ (2020) Exposure to endophytic fungi quantitatively and compositionally alters anthocyanins in grape cells. Plant Physiol Biochem 149:144–152. https://doi.org/10.1016/j.plaphy.2020.02.006
Yuan J, Zhang W, Sun K, Tang MJ, Chen PX, Li X, Dai CC (2019) Comparative transcriptomics and proteomics of Atractylodes lancea in response to endophytic fungus Gilmaniella sp. AL12 reveals regulation in plant metabolism. Front Microbiol 10:1208. https://doi.org/10.3389/fmicb.2019.01208
Zuccaro A, Basiewicz M, Zurawska M, Biedenkopf D, Kogel KH (2009) Karyotype analysis, genome organization, and stable genetic transformation of the root colonizing fungus Piriformospora indica. Fungal Genet Biol 46:543–550. https://doi.org/10.1016/j.fgb.2009.03.009
Zuccaro A, Lahrmann U, Güldener U, Langen G, Pfiffi S, Biedenkopf D, Wong P, Samans B, Grimm C, Basiewicz M, Murat C, Martin F, Kogel KH (2011) Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLoS Pathog 7:e1002290. https://doi.org/10.1371/journal.ppat.1002290
Acknowledgements
This project was supported by the Austrian Science Fund (FWF) Grant: P 29620-B25. We acknowledge Tina Austerlitz, Sabine Daxböck-Horvath and Karin Baumgartner for general maintenance of the laboratory.
Funding
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Opitz, M.W., Daneshkhah, R., Lorenz, C. et al. Serendipita indica changes host sugar and defense status in Arabidopsis thaliana: cooperation or exploitation?. Planta 253, 74 (2021). https://doi.org/10.1007/s00425-021-03587-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03587-3